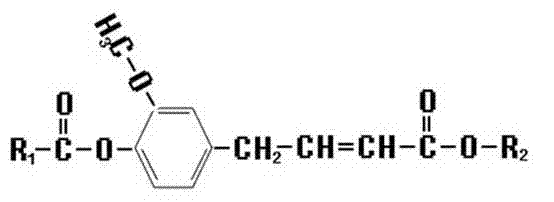
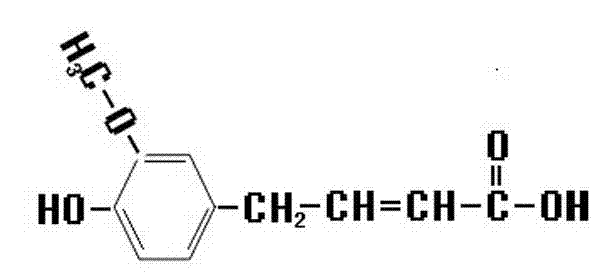
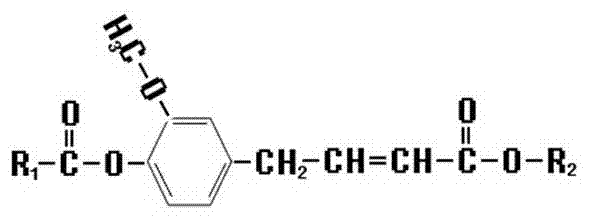
Novel ferulic acid derivative, its application and synthetic method
A synthetic method, the technology of ethyl ferulate, applied in the direction of chemical instruments and methods, drug combinations, medical preparations containing active ingredients, etc., can solve the problem of inability to solve stability, affect the therapeutic effect of the product, and attenuate the antioxidant capacity and other problems, to achieve the effect of strong expansion of blood vessels, strong antioxidant capacity, and strong anticoagulant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] Implementation example one:
[0029] Synthetic method of ethyl acetyl ferulate
[0030] Put 10~20g of ethyl ferulate and 30~100ml of tetrahydrofuran in a reaction vessel, stir, add 8~20g of anhydrous sodium carbonate after completely dissolving, and weigh 5~15g of acetic anhydride in a funnel, add slowly in a normal temperature water bath Acetic anhydride, stirred, heated up to 30~55°C and reacted for 0.5~2 hours, added 30~100ml of purified water, stirred, collected the organic layer, filtered the solid with suction and dried to obtain ethyl acetyl ferulate. Its synthetic reaction formula is as follows:
[0031] C 12 h 15 o 4 (Ethyl ferulate) + C 4 h 6 o 3 (Acetic anhydride) → C 2 h 4 o 2 (Acetic acid) +C 14 h 17 o 5 (Ethyl Acetyl Ferulate)
[0032] CH 3 COOH (acetic acid) + Na 2 CO 3 (sodium carbonate) → NaHCO 3 (sodium bicarbonate) + CH 3 COONa (sodium acetate)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com