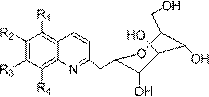
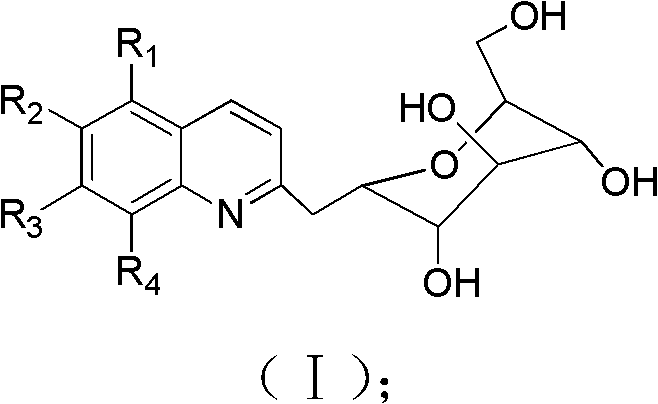
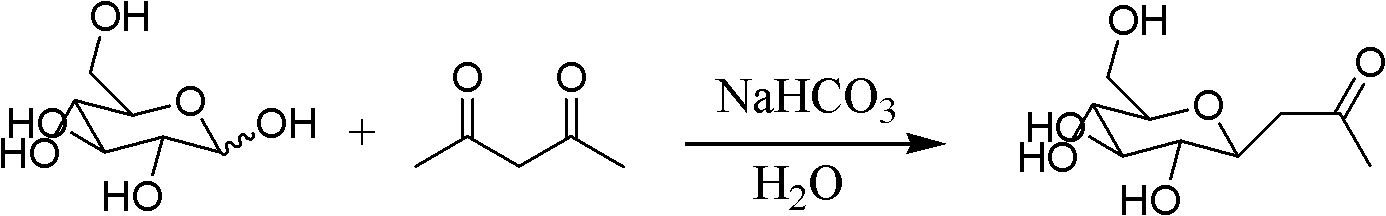Application of 2-gylcosyl chinoline compound in preparing acetylcholine esterase resisting medicines
The technology of acetylcholinesterase and glycosyl quinoline is applied in the application field of 2-glycosyl quinoline compounds in the preparation of anti-acetylcholinesterase drugs, and achieves the effects of high bioavailability, strong solubility and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The synthesis of embodiment 1 intermediate β-D-acetonyl glucoside
[0025] Preparation route:
[0026]
[0027] Weigh 1~5g of D-glucose and 0.5~2.5g of sodium bicarbonate into a round bottom flask, add distilled water and stir at room temperature for 10~30min, then add 1~5g of acetylacetone and stir in an oil bath at 50~120℃ Under the reaction 2 ~ 10h. Stop the reaction, cool and then extract with carbon dichloride, adjust the pH to 7 with dilute hydrochloric acid, remove water under reduced pressure and then add methanol, the inorganic salt precipitates because it is insoluble in methanol, and can be removed by filtration (or through a silica gel column). The product can be obtained by removing the solvent under reduced pressure. The yield is about 77%, m.p: 122-124°C.
Embodiment 2
[0028] Embodiment 2, the synthesis of compound A1
[0029] Preparation route:
[0030]
[0031] Weigh 0.5 mmol of 2-aminobenzaldehyde and 0.5 mmol of β-acetonyl glucoside into a round bottom flask, add methanol, and stir at room temperature to dissolve them. Add 25 mol% pyrrolidine (relative to 2-aminobenzaldehyde), and follow the reaction with TLC in an oil bath at 60-120°C until the reaction remains unchanged. The reaction was stopped and cooled, and the solvent in the reaction was removed under reduced pressure, and the pure product was obtained by passing through the column with ethyl acetate:isopropanol:water=16:2:1 (v / v / v).
[0032] White solid; m.p: 257-258°C; IR (KBr, cm -1 ): 3482(vs), 3385(s), 3329(s), 3104(m), 2908(m), 1601(m), 1563(w), 1427(m), 1298(s), 1127(m ), 1088(vs), 1033(s), 838(m), 763(m); 1 H NMR (DMSO-d 6 ): δ8.23(dd, J=2.8Hz, J=5.2Hz, 1H, Ar-H), 7.94(t, J=7.2Hz, 2H, Ar-H), 7.72(s, 1H, Ar-H ), 7.55(d, J=8.0Hz, 2H, Ar-H), 5.20(s, 1H), 5.00(s, 1H),...
Embodiment 3
[0033] The synthesis of embodiment 3 compound A2
[0034] The preparation method is the same as in Example 2, except that 3,6-dimethoxy-2-aminobenzaldehyde is used instead of 2-aminobenzaldehyde to obtain compound A2.
[0035]
[0036] White solid; m.p: 121-122°C; IR (KBr, cm -1 ): 3522(s), 3268(s), 2871(s), 1619(s), 1604(s), 1346(s), 1263(vs), 1117(vs), 1090(vs), 1043(s) ), 908(m), 723(m); 1 H NMR (DMSO-d 6 ): δ8.33(s, 1H, Ar-H), 7.52(s, 1H, Ar-H), 7.02(s, 1H, Ar-H), 6.85(s, 1H, Ar-H), 5.25( s, 1H), 4.96(s, H), 4.88(s, 1H), 4.30(s, 1H), 4.15(s, 1H), 3.89(s, 3H), 3.88(s, 3H), 3.17(s , 2H), 3.09-2.97(m, 3H), 2.87(d, J=8.0H, 1H);13 C NMR (DMSO-d 6 ): δ164.6, 153.9, 153.2, 144.5, 135.0, 127.5, 124.4, 113.1, 108.7, 85.7, 84.5, 79.3, 75.5, 66.4, 60.9, 60.8.0, 46.5; ESI-MS m / z calcd for C 18 h 23 NO 7 ([M+1] + ): 365.15, Found: ([M+1] + ): 366.44, ([M+23] + ): 388.32, ([2M+23] + ): 752.87; Anal.Calcd for C 18 h 23 NO 7 : C, 59.17; H, 6.34; N, 3.83; Found: C, 59.25...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com