Novel gentisic acid derivatives (2-hydroxy-5-alkyl (H) oxo benzoates) as well as preparation method and new application thereof
A technology of methyl gentisate and oxybenzoic acid, which is applied in the field of skin whitening, can solve problems such as unsuitable whitening agents, and achieve the effects of simple process route, obvious social and economic benefits, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
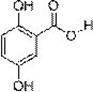
[0020] Example 1: Novel gentisic acid derivatives 2-hydroxy-5-alk(H)oxybenzoates, the structural formula of which is:
[0021]
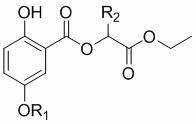
[0022] In the structural formula, R 1 Any one of hydrogen, methyl, ethyl, benzyl and p-nitrobenzyl, R 2 is hydrogen or methyl.
[0023] The novel gentisic acid derivative 2-hydroxy-5-alk(H)oxybenzoate is used in the field of skin whitening.
[0024] A method for preparing novel gentisic acid derivatives 2-hydroxy-5-alk(H)oxybenzoates, including methyl gentisate, halogenated hydrocarbons, potassium carbonate, sodium hydroxide and α-hydroxy acid esters;
[0025] The molar ratio of methyl gentisate to halogenated hydrocarbons and potassium carbonate is 1:1.2:1.5, the molar ratio to sodium hydroxide is 1:2~5, and the molar ratio to α-hydroxy acid ester is 1 :2;
[0026] First, add methyl gentisate and anhydrous acetone in sequence in the container (the ratio of methyl gentisate to anhydrous acetone is 1:0.2~1), add halogenated hydrocarbon under st...
Embodiment 2
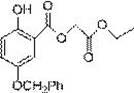
[0049] Embodiment 2: This embodiment prepares ethoxycarbonylmethyl 2-hydroxy-5-methoxybenzoate (6a for short), and its structural formula is as follows:
[0050]
[0051] The processing steps of the present embodiment are as follows:
[0052] Preparation of ethoxycarbonylmethyl 2-hydroxy-5-methoxybenzoate (abbreviated as 6a)
[0053] Take 50 mg (0.30 mmol) of pure methyl gentisate, dissolve it in 10 mL of acetone, and then add 62 mg (0.45 mmol) of K 2 CO 3 , 0.51g (0.36mmol) CH 3I, the reaction was stirred under reflux, followed by TLC until the reaction was complete, filtered, the filtrate was concentrated under reduced pressure, and the crude product was separated and purified by column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain 2-hydroxy-5-methoxybenzene Methyl formate. Take 50mg (0.27mmol) of methyl 2-hydroxy-5-methoxybenzoate, dissolve it in 10ml of 95% methanol solution, and slowly add NaOH solid under stirring at room temperature, then raise ...
Embodiment 3
[0054] Embodiment 3: This embodiment prepares ethoxycarbonylmethyl 2-hydroxy-5-ethoxybenzoate (6b for short), and its structural formula is as follows:
[0055]
[0056] The processing steps of the present embodiment are as follows:
[0057] Preparation of ethoxycarbonylmethyl 2-hydroxy-5-ethoxybenzoate (6b for short):
[0058] Take 50 mg (0.30 mmol) of pure methyl gentisate, dissolve it in 10 mL of acetone, and then add 62 mg (0.45 mmol) of K 2 CO 3 , 0.39g (0.36mmol) BrCH 2 CH 3 , reflux and stir the reaction, TLC traced until the reaction was complete, filtered, the filtrate was concentrated under reduced pressure, and the crude product was separated and purified by column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain 2-hydroxy-5-ethoxybenzoic acid methyl ester. Take 50mg (0.26mmol) of methyl 2-hydroxy-5-ethoxybenzoate, dissolve it in 10ml of 95% methanol solution, and slowly add NaOH solid under stirring at room temperature, then raise the tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com