Improved method for synthetizing novel P38 mitogen-activated protein kinase inhibitor
A protein kinase inhibitor, mitogen activation technology, applied in the field of improved synthesis, can solve the problems of unsuitable environmental pollution, high toxicity, expensive reducing agent, etc., achieve simplified reaction steps, simple purification method, and overcome environmental pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of Compound C
[0045] Compound B (3Kg, 23.786mol), 16Kg of THF and N-benzyloxycarbonylglycine (5Kg, 23.9mol) were sequentially added into a 100L reactor. Control the temperature of the reaction solution at 35-40°C and add HOBT (0.63Kg, 4.662mol) in batches; 3Kg of DMF is added at one time. At the same temperature, EDCI (5Kg, 26.08mol) was added in batches; keep warm and stir the reaction for 10h. Add 18Kg of deionized water in batches. At a temperature below 65 °C, the reaction solvent was distilled to about 20 L. At a temperature of 35-40°C, add 35Kg of deionized water in batches. Centrifuge and dry to obtain the crude product, which is washed twice with deionized water, 30Kg each time. Vacuum drying at 50°C gave 6.83Kg of compound C (90.5%, molar yield).
[0046] 1 H-NMR(DMSO-d6)δ: 13.465(s, 1H), 8.273(s, 1H), 8.259(s, 1H), 7.257~7497(m, 5H), 6.861~6.867(d, 1H), 5.079 (s, 2H), 4.595 ~ 4.611 (d, 2H).
[0047] Preparation of Compound D
[0048] Com...
Embodiment 2
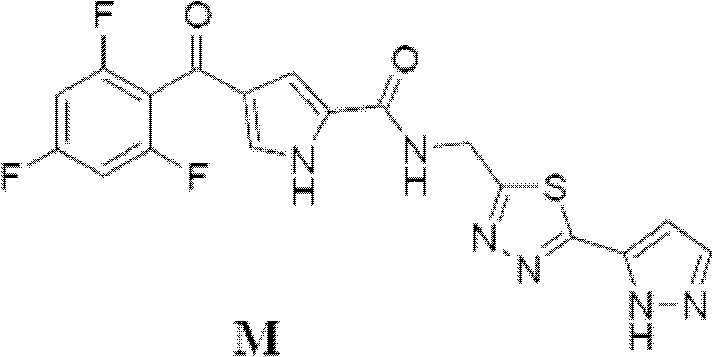
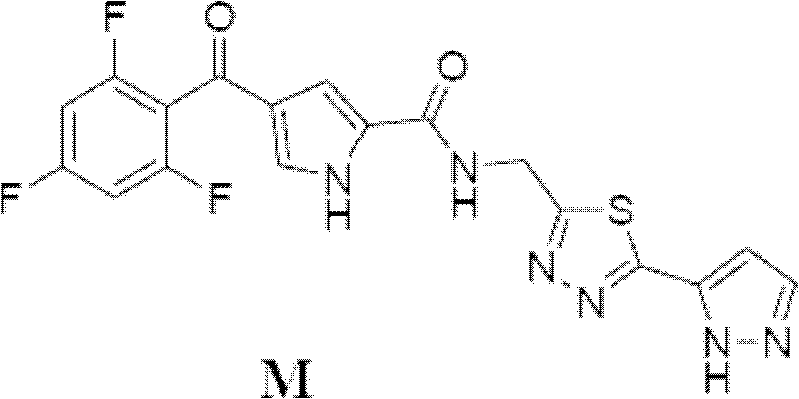
[0057] The preparation method of compound C, D, E is with embodiment 1, and the preparation method of compound M is roughly the same as the method of embodiment 1, and difference is that the condensing agent that adopts is TBTU:
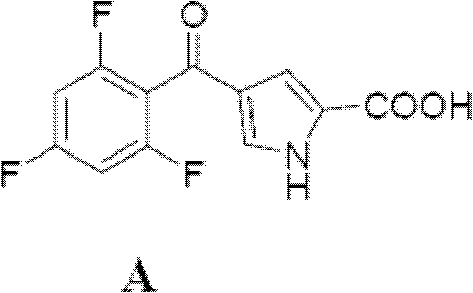
[0058] Compound E (562g, 2.145mol), 10Kg of THF, Compound A (570g, 2.118mol) and TBTU (815g, 2.535mol) were sequentially added into a 50L reactor. Control the temperature at 35° C., and add 527 g of triethylamine in batches. The reaction solution was kept at 60-65°C and stirred for 20h. The reaction liquid was cooled to 25°C, and 6.5Kg of water was added dropwise while controlling the temperature of the reaction liquid at 20-25°C. Under heat preservation, stir for 30 minutes, centrifuge to dry, and wash the solid twice with 10Kg deionized water successively. 1.2Kg of wet compound M was obtained.
[0059] Add 1.2Kg of wet compound M, 3.5Kg of DMF and 5Kg of THF into a 10-degree kettle in sequence, and stir to dissolve the solid. Suction filter the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com