Preparation of fatty acid-RGD-fatty alcohol conjugate mediated epirubicin targeted liposome and evaluation on anticancer activity
A technology targeting liposomes and fatty acids, which is applied in the field of biomedicine and can solve the problems that ordinary liposomes do not have targeting properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1CH 3 (CH 2 ) 10 CO-Arg-Gly-Asp-OCH 2 (CH 2 ) 10 CH 3 preparation of
[0045] 1) Preparation of Boc-Arg(NO2)-Gly-OBzl
[0046] 1.59 g (5 mmol) Boc-Arg(NO2)-OH, 0.68 g (5 mmol) N-hydroxybenzotriazole (HOBt) were dissolved in anhydrous THF. 1.34 g (5.75 mmol) of anhydrous THF solution of dicyclohexylcarbodiimide (DCC) was added dropwise under ice bath, and stirred for 20 minutes under ice bath to obtain reaction solution (I). Suspend 1.69g (5.0mmol) TosOH·Gly-OBzl in an appropriate amount of anhydrous DMF under ice-cooling, then add a few drops of N-methylmorpholine (NMM) to adjust the pH to 7-8 to obtain the reaction solution (II) . Add the reaction solution (I) dropwise to the reaction solution (II) under ice bath, first stir under ice bath for 1 h, then stir at room temperature for 12 h, TLC (chloroform / methanol, 10:1) shows that Boc-Arg(NO2)-OH disappears , stop responding. Dicyclohexylurea (DCU) was filtered off, and the filtrate was blown off of D...
Embodiment 2
[0062] Embodiment 2. The preparation of epirubicin liposome (2)
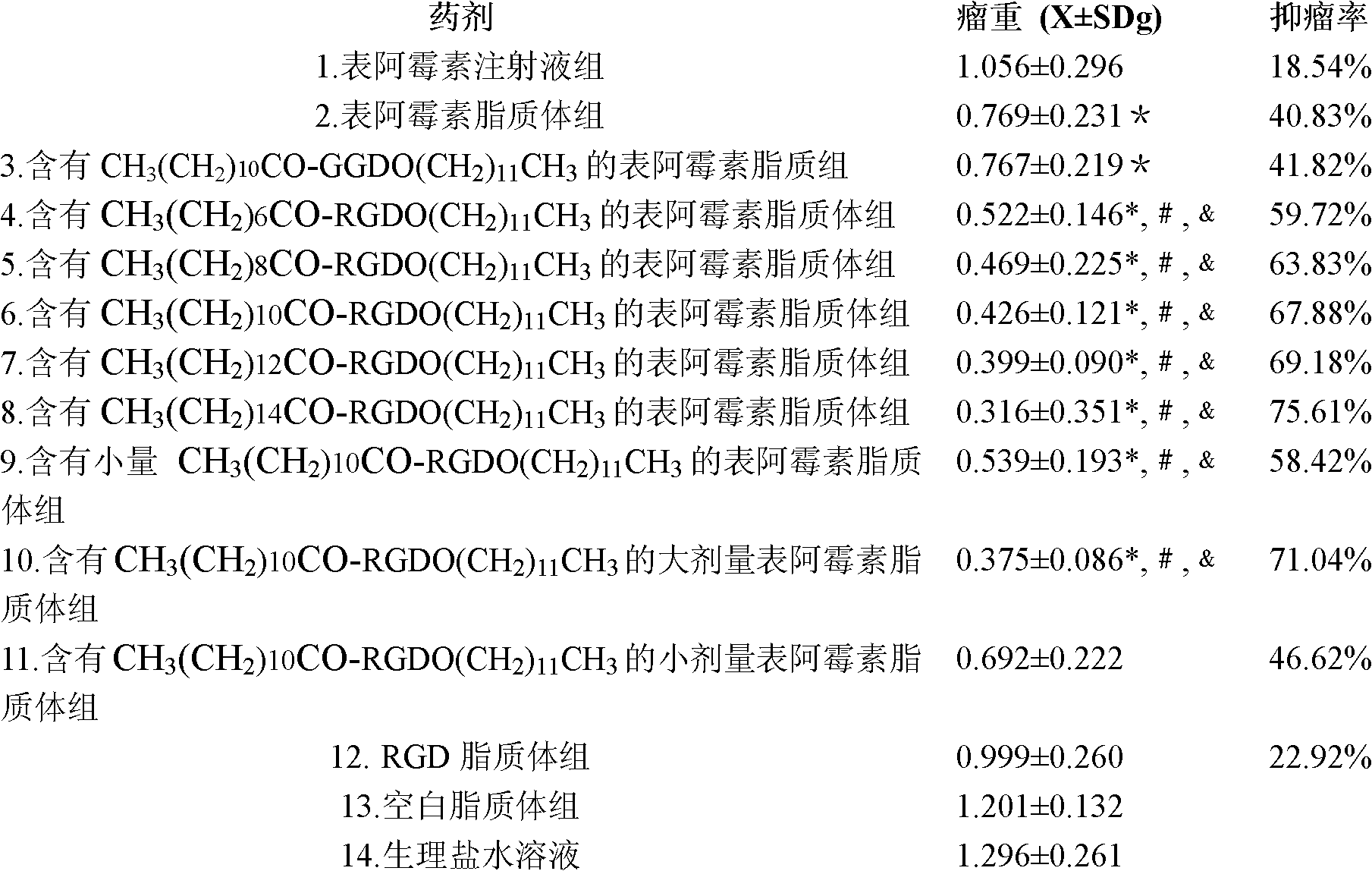
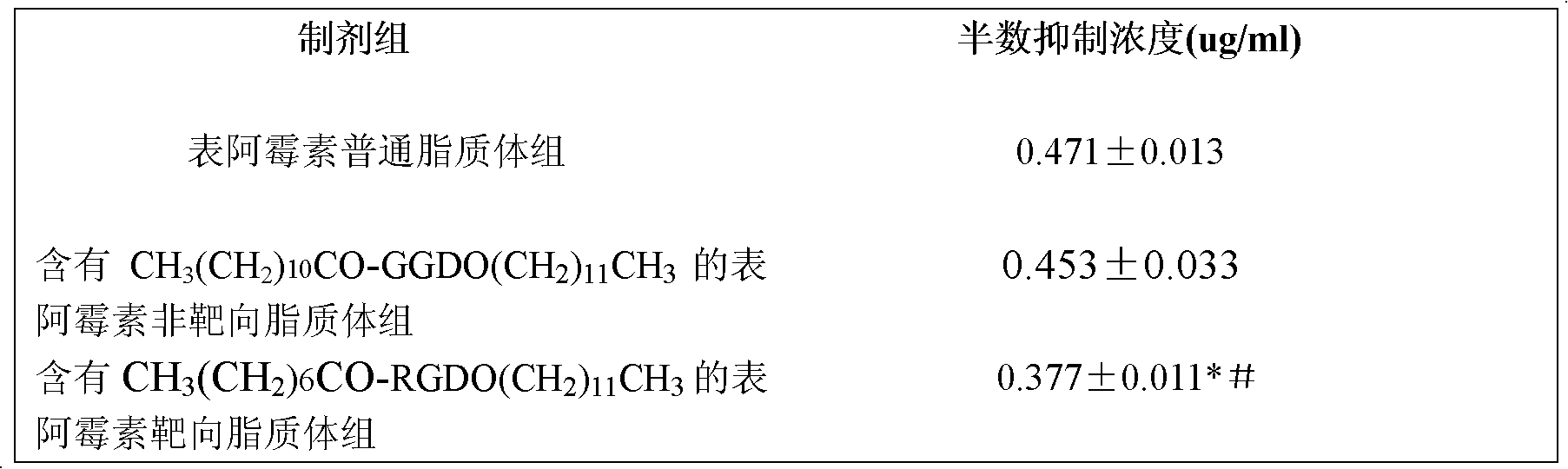
[0063] Prepared by ammonium sulfate gradient method. Take an appropriate amount of phospholipids and cholesterol into a round bottom flask and dissolve them in chloroform, and remove the solvent by rotary evaporation, forming a transparent film on the bottle wall. 150mmol·L -1 (NH 4 ) 2 SO 4 The solution was added to the lipid film, and ultrasonically dispersed to obtain a blank liposome suspension. Put the blank liposome in the dialysis bag for dialysis 4-5 times, each time for about 10 hours, take out the dialysis liquid and place it in an eggplant bottle; in addition, accurately weigh an appropriate amount of epirubicin, dissolve it in water, and add this solution to Place the blank liposome suspension in a hot water bath and shake it from time to time. After taking out from the hot water bath, the epirubicin liposome is obtained.
[0064] Particle size 150~250nm, Zeta-Potential-20~-40mV.
Embodiment 3
[0065] Example 3. Containing CH in the membrane material 3 (CH 2 ) 10 CO-GGD-OCH 2 (CH 2 ) 10 CH 3 Preparation of epirubicin liposomes (3)
[0066] The dosage of each component: epirubicin 2-8%; natural lecithin, cholesterol and CH 3 (CH 2 ) 10 CO-GGD-OCH 2 (CH 2 ) 10 CH 3 The mixture 90-97%, in this mixture, natural lecithin 70-80% (mass percentage), cholesterol 10-20% (mass percentage) and compound CH 3 (CH 2 ) 10 CO-GGD-OCH 2 (CH 2 ) 10 CH 3 3-10% (mass percentage).
[0067] Take phospholipids, cholesterol and CH 3 (CH 2 ) 10 CO-GGD-OCH 2 (CH 2 ) 10 CH 3 Dissolve in chloroform in a round-bottomed flask, remove the solvent by rotary evaporation, and form a transparent film on the bottle wall. 150mmol·L -1 (NH 4 ) 2 SO 4 The solution was added to the lipid film, and ultrasonically dispersed to obtain a blank liposome suspension. Place the blank liposome in the dialysis bag for dialysis 4-5 times, each time for about 10 hours, take out the dialy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com