Self-emulsifying doxorubicin nanometer medicament and preparation method thereof
A nano-drug, doxorubicin technology, applied in the preparation of sugar derivatives, chemical instruments and methods, pharmaceutical formulations, etc., can solve the problem of inability to effectively use the EPR effect to target delivery of DOX, inability to effectively form nano-drugs, difficult to eliminate In vitro and other problems, to achieve the effect of long blood retention time, long-lasting and stable blood concentration, and high cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
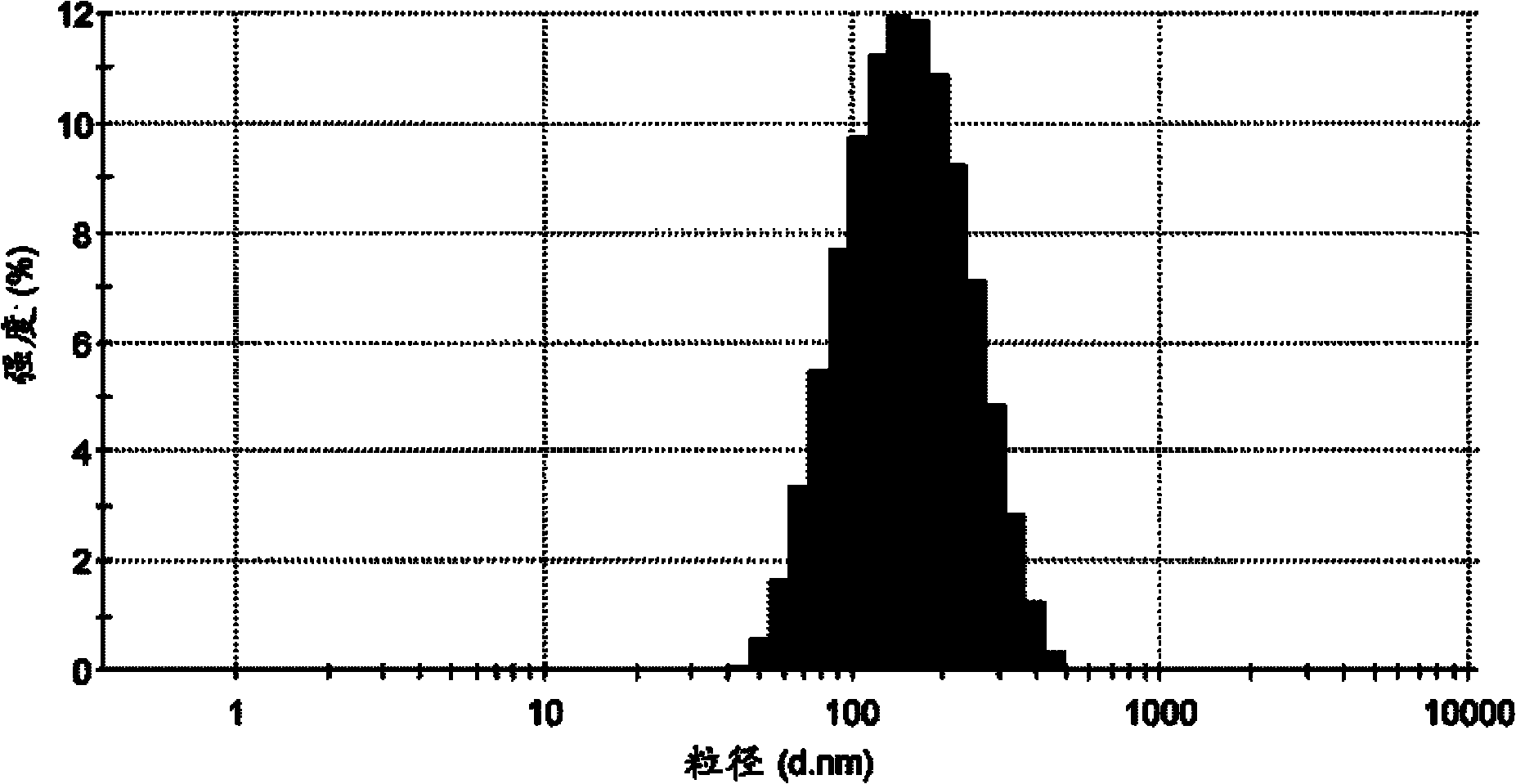
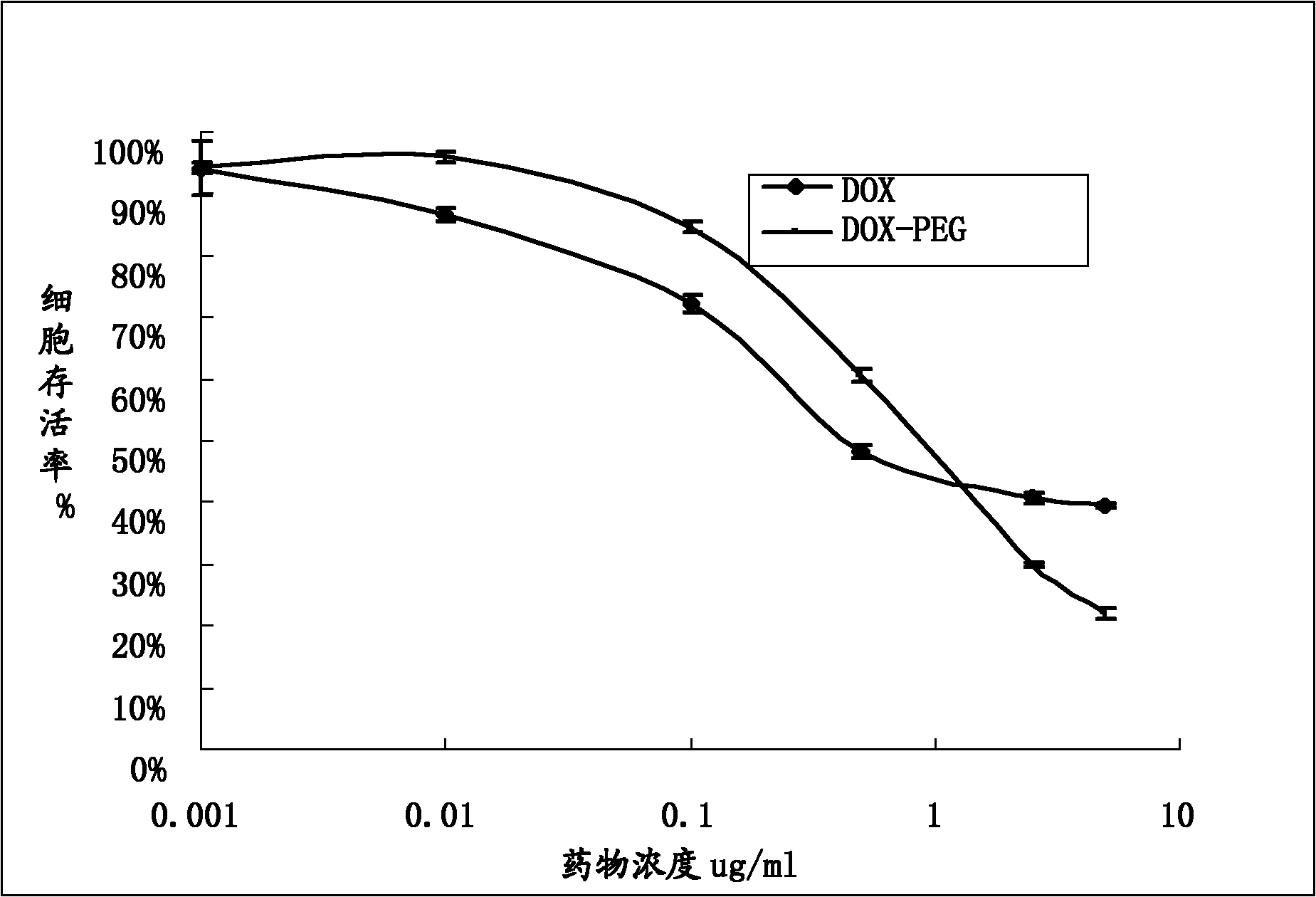
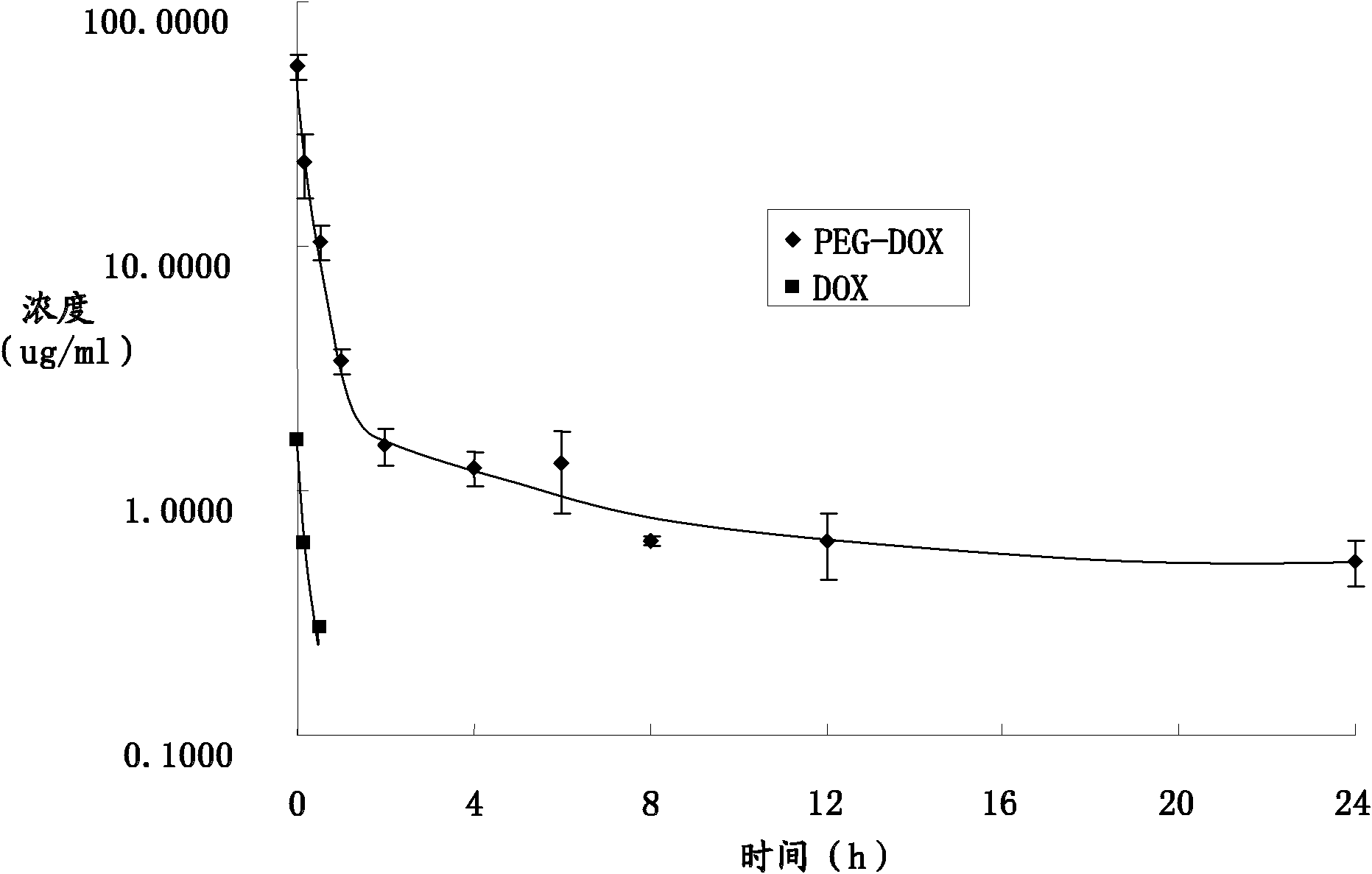
Image
Examples
Embodiment 1
[0037] The self-emulsifying doxorubicin nanomedicine PEG-DOX was formed by linking PEG and the carbonyl group of DOX with a hydrazone bond.
[0038] (1) Compound a (PEG-CH 2 Synthesis of COOEt)
[0039] PEG-OH (Mn=550, 10.2154g) was added to the oven-dried three-necked flask, heated and vacuum-dried, THF30ml after the potassium metal reflux was added to remove water, and NaH (Mn=24, 0.6685g) was added to it quickly. , react until no bubbles are produced, centrifuge, and take the supernatant. Then, ethyl bromoacetate (Mn=167, 3.4120 g, 2.275 ml) was added to the supernatant, and a large amount of white precipitate was formed immediately, and the reaction was terminated after 2 h. THF was removed by rotary evaporation, a small amount of water was added, and CH 2 Cl 2 Extraction and take the organic phase. The organic phase was rotary evaporated to remove most of the CH 2 Cl 2 , adding n-hexane for precipitation, repeating this for many times, and then rotary evaporation o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com