Detection method for agglutination test of rabies virus neutralizing antibody cells
A rabies virus and antibody technology, applied in the field of bioengineering, can solve the problems of high detection cost, complicated operation and long time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Construction of Eukaryotic Expression Vector of Rabies Virus Glycoprotein Gene
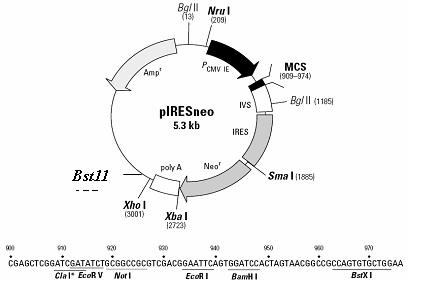
[0030] Using the commercially available eukaryotic expression plasmid vector pIRES-neo ( figure 1 ) polyclonal restriction site EcoR V and Bam H I inserted the rabies virus glycoprotein gene (GenBank: AF499686.2) to construct an expression cassette expressing the glycoprotein gene.
[0031] EcoR V and Bam H I double enzyme digestion system (product of Treasure Bioengineering (Dalian) Co., Ltd.): 10 U of each of the two endonucleases, 2 μl of K buffer, 1 μg of pIRES-neo plasmid, and make up to 20 μl of distilled water. After bathing in water at 37°C for 1 hour, a 5.3bp band was recovered by 1% agarose gel electrophoresis (for the recovery step, refer to the instructions of the DNA gel recovery kit from Axygen).
[0032] DNA ligation system (product of Treasure Bioengineering (Dalian) Co., Ltd.): T4 DNA ligase 200U, reaction buffer 1 μl, rabies virus glycoprotein gene fragment...
Embodiment 2
[0036] Establishment of detection method of human rabies virus neutralizing antibody cell agglutination test
[0037] 1. Establishment of Stable Expression Cell Lines
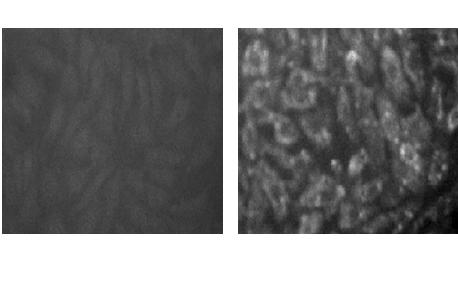
[0038] Under the mediation of liposome 2000, the constructed eukaryotic expression vector pIRES-neo / G was transfected into human embryonic kidney cells 293. Transfection step: take 10 μl liposome 2000, mix it with 5 μg pIRES-neo / G in equal volume for 5 minutes; remove the cell culture medium, spread the mixture on the monolayer cells, 37°C 5% CO 2 Induction for 30min, add DMEM medium containing 5% calf serum, 37°C 5%CO 2 nourish. The next day, the cell culture medium was removed, and the cells were screened with DMEM containing 150 μg / mL G418 and 5% calf serum to obtain the 293 cell line stably expressing the neo gene. The obtained cells were stained with commercial FITC-labeled rabies virus antibody, and the expression of glycoprotein was identified under a fluorescent microscope ( figure 2 ).
[0039]...
Embodiment 3
[0053] Establishment of detection method of canine rabies virus neutralizing antibody cell agglutination test
[0054] 1. Establishment of stable expression cell lines
[0055] Under the mediation of liposome 2000, the constructed eukaryotic expression vector pIRES-neo / G was transfected into canine kidney cells MDCK. Transfection step: take 10 μl liposome 2000, mix it with 5 μg pIRES-neo / G in equal volume for 5 minutes; remove the cell culture medium, spread the mixture on the monolayer cells, 37°C 5% CO 2 Induction for 30min, add DMEM medium containing 5% calf serum, 37°C 5%CO 2 nourish. The next day, the cell culture medium was removed, and the cells were screened with DMEM containing 300 μg / mL G418 and 5% calf serum to obtain the MDCK cell line stably expressing the neo gene. The obtained cells were stained with commercial FITC-labeled rabies virus antibody, and the expression of glycoprotein was identified under a fluorescent microscope ( Figure 4 ).
[0056] 2. Pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com