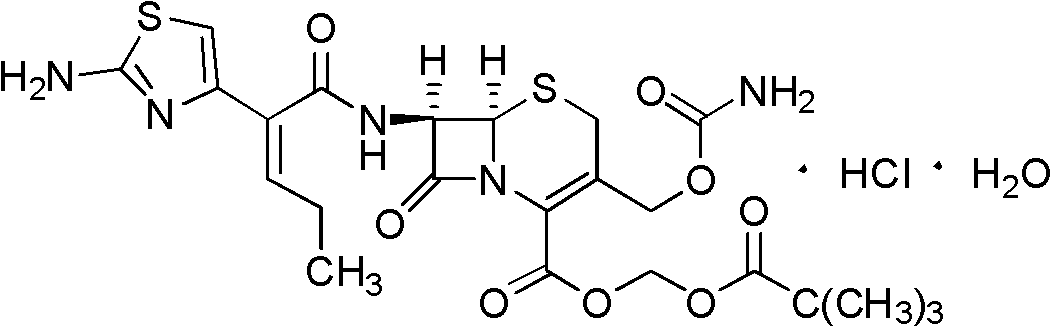
Cefcapene pivoxil hydrochloride hydrate composition tablets
A technology of cefcapine and a composition, which is applied in the field of medicine, can solve the problems of affecting drug efficacy, tablet hardness and tablet drug efficacy being difficult to satisfy at the same time, affecting tablet absorption and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 The cefcarpine pivoxil hydrochloride composition tablet provided by the present invention
[0036] Accurately weigh 100 mg of cefcarpine pivoxil hydrochloride, 30 mg of microcrystalline cellulose, 22 mg of mannitol, 22 mg of hyprolose, 1.5 mg of magnesium stearate, and 0.5 mg of lactose;
[0037] Described microcrystalline cellulose, mannitol, lactose are pulverized respectively and crossed 80 mesh sieves, respectively crossed 100 mesh sieves by cefcapine hydrochloride pivoxil, hydroxypropyl cellulose, magnesium stearate; Ester, 30mg of microcrystalline cellulose, 22mg of mannitol and 11mg of hydroxypropyl cellulose were mixed and crushed and sieved to collect 24 mesh particles, and 11mg of hydroxypropyl cellulose and 1.5mg of magnesium stearate were added, and after mixing, The cefcarpine pivoxil hydrochloride tablet was obtained after tabletting; the Opadry coating powder of 4.4 mg was made into a 4% coating with a mass fraction of 80% ethanol of 55 mg. ...
Embodiment 2
[0038] Embodiment 2 The cefcarpine pivoxil hydrochloride composition tablet provided by the present invention
[0039] Accurately weigh 100 mg of cefcarpine pivoxil hydrochloride, 30 mg of microcrystalline cellulose, 22 mg of mannitol, 22 mg of hyprolose, 1.5 mg of magnesium stearate, and 0.5 mg of lactose;
[0040] Described microcrystalline cellulose, mannitol, lactose are pulverized respectively and crossed 80 mesh sieves, respectively crossed 100 mesh sieves by cefcapine hydrochloride pivoxil, hydroxypropyl cellulose, magnesium stearate; Ester, 30mg of microcrystalline cellulose, 22mg of mannitol and 11mg of hydroxypropyl cellulose were mixed and crushed and sieved to collect 24 mesh particles, and 11mg of hydroxypropyl cellulose and 1.5mg of magnesium stearate were added, and after mixing, Acid cefcarpine pivoxil composition is obtained, cefcarpine pivoxil hydrochloride tablet is obtained after tabletting; the Opadry coating powder of 4.4mg is made the mass fraction of 6%...
Embodiment 3
[0041] Embodiment 3 Cefcarpine pivoxil hydrochloride composition tablet provided by the present invention
[0042] Accurately weigh 100 mg of cefcarpine pivoxil hydrochloride, 30 mg of microcrystalline cellulose, 22 mg of mannitol, 22 mg of hyprolose, 1.5 mg of magnesium stearate, and 0.5 mg of lactose;
[0043] Described microcrystalline cellulose, mannitol, lactose are pulverized respectively and crossed 80 mesh sieves, respectively crossed 100 mesh sieves by cefcapine hydrochloride pivoxil, hydroxypropyl cellulose, magnesium stearate; Ester, 30mg of microcrystalline cellulose, 22mg of mannitol and 11mg of hydroxypropyl cellulose were mixed and crushed and sieved to collect 24 mesh particles, and 11mg of hydroxypropyl cellulose and 1.5mg of magnesium stearate were added, and after mixing, Obtained acid cefcarpine pivoxil composition, made cefcarpine pivoxil hydrochloride tablet after tabletting; The Opadry coating powder of 4.4mg is made the coating that mass fraction is 8% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com