Method for treating septicemia with human neutrophil peptide (HNP) blocking agents
A technology of neutrophils and blocking agents, applied in the direction of antibacterial drugs, anti-animal/human immunoglobulins, antibodies, etc., can solve the problems of reducing the production, function and biological activity of neutrophil HNP, and achieve The effect of enhancing the therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
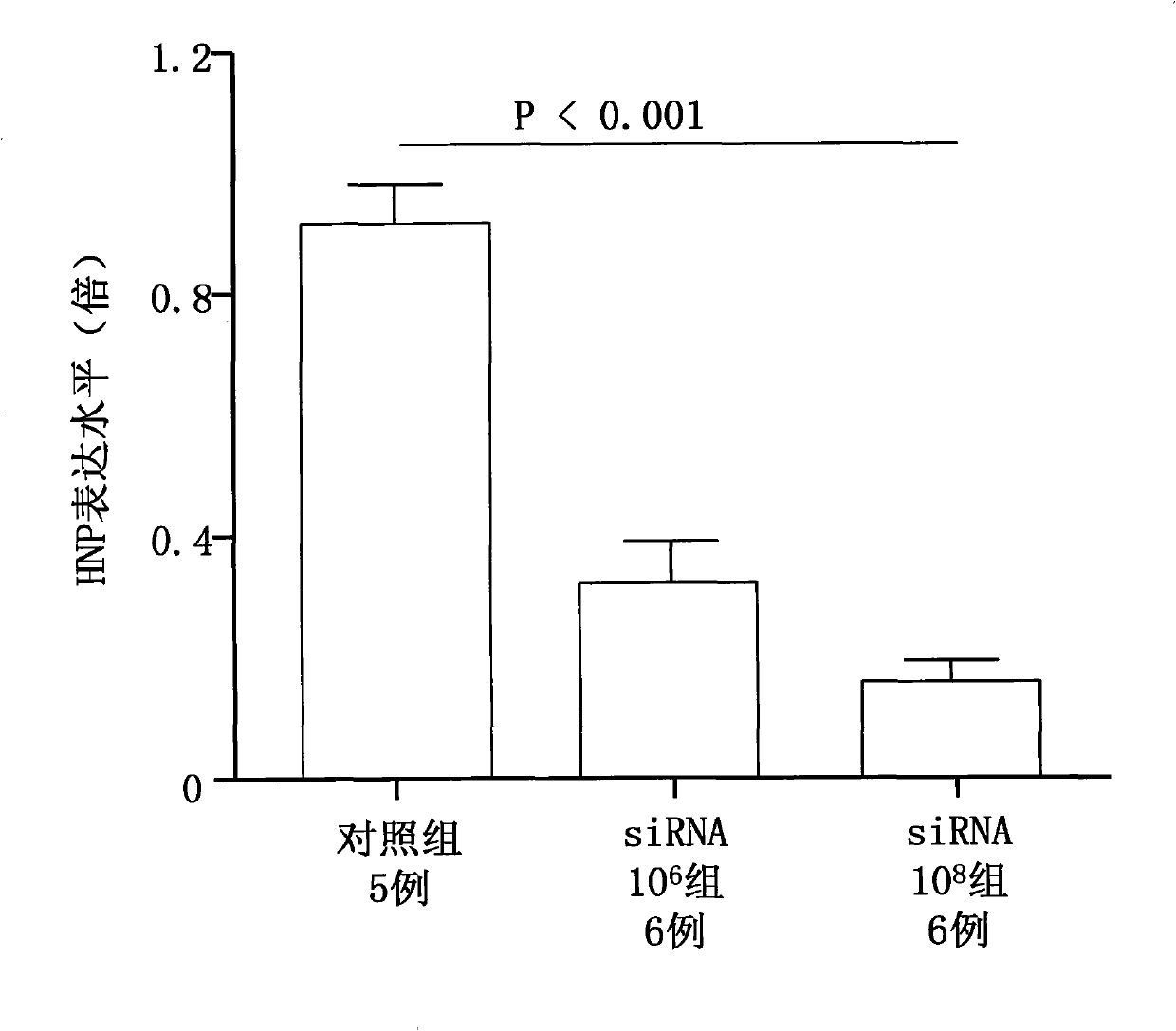
[0019] In order to efficiently express siRNA, a lentiviral vector system is used, because lentiviral vectors have a wider host range than retroviral vectors. The gene sequence AGGCACTGCTCTCCAAGAT was assembled into a self-developed lentiviral vector. The lentiviral vector and other vector components are co-introduced into human 293T cells in order to replicate and express the lentivirus. After 48 hours, the lentivirus titer reached 1×10 per mL 13 Plaque forming units (pfu).
[0020] In order to test that the above siRNA can effectively inhibit the expression of HNP, bone marrow cells were prepared from rat bone marrow. Two Wistar rats were sacrificed by cervical dislocation. The whole body was sterilized with 75% alcohol, and a total of 4 femurs were taken. All bone marrow cells were washed out with RPMI1640 (pH7.2) in a sterile environment. Pipet 5 times with a pipette, and pass through a No. 4 needle 5 times to make a single-cell suspension. Centrifuge at 1000 rpm for ...
Embodiment 2
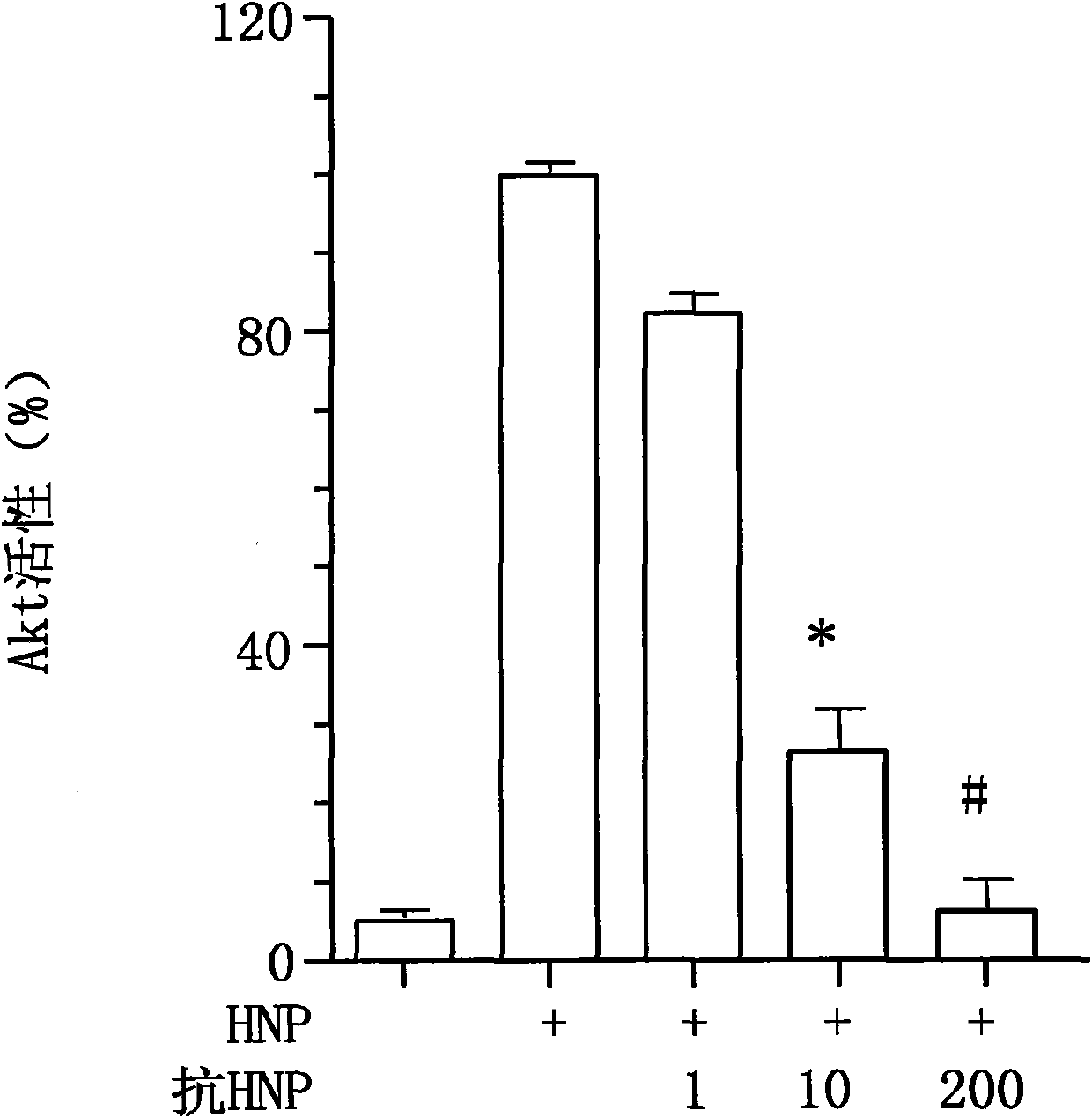
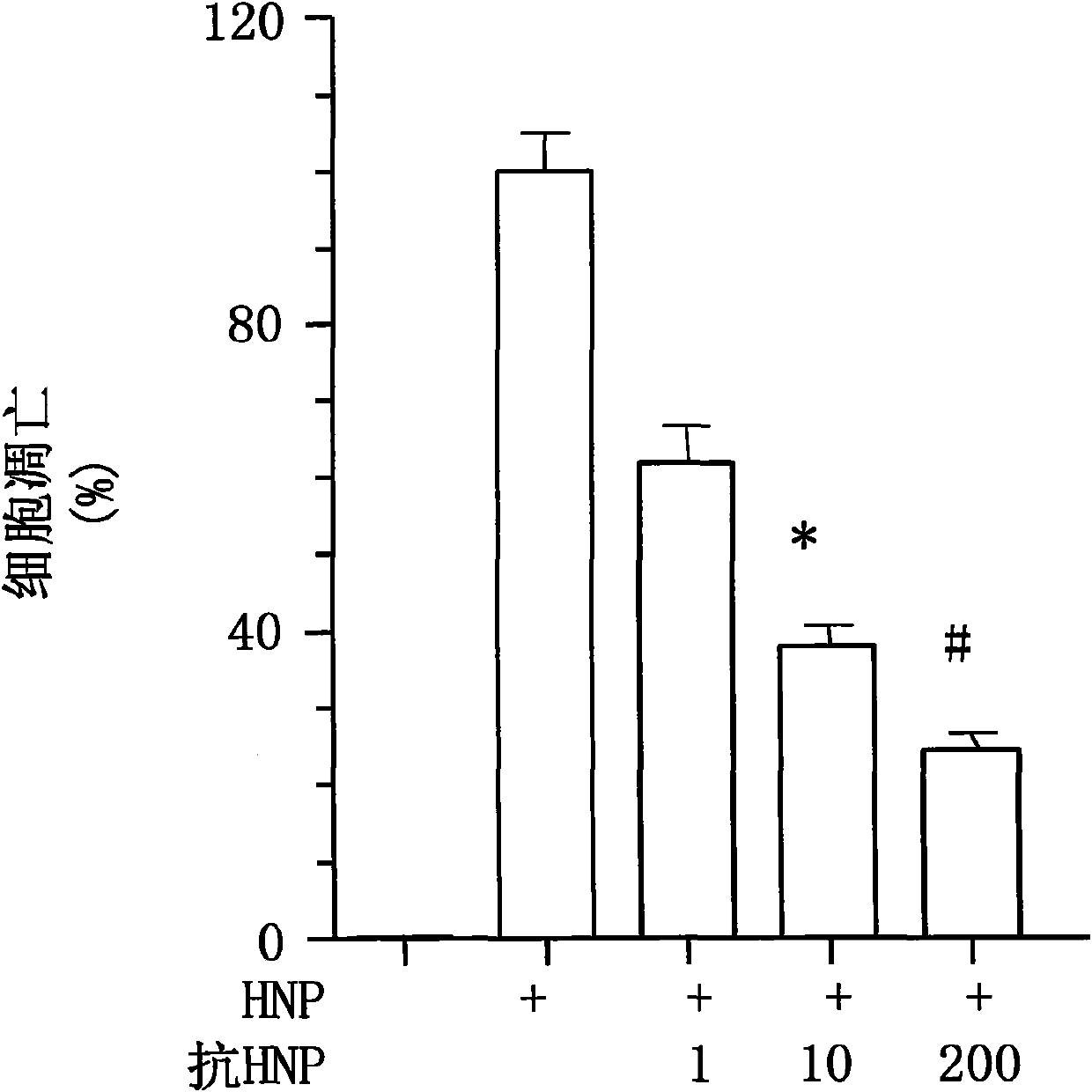
[0022]In order to clarify that anti-human HNP-1 antibody can effectively neutralize the activity of extracellular HNP in neutrophils, two experiments were performed. In the first trial, complex HNP (70% HNP-1, 21% HNP-2 and only 5% HNP-3 were extracted from the mixed sputum of 30 patients with chronic bronchopneumonia, with no detectable HNP- 4) [Refer to literature Khine, et al., Blood 107, 2936-2942 (2006)]. 100 μg / ml HNP complex and anti-human HNP-1 antibody (1, 10 and 200 μg / ml) were incubated at room temperature for 30 minutes, and the mixture was used to act on lung cancer A549 cells at 10 μg / ml, and the cells were lysed after 30 minutes. The activity of Akt in cells was detected by immunoenzyme labeling. The results show that anti-human HNP-1 antibody can block HNP-activated Akt enzyme activity in lung cancer A549 cells [see figure 2 】. In the second experiment, 100 μg / ml HNP complexes extracted from human sputum were incubated with anti-human HNP-1 antibodies (1, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com