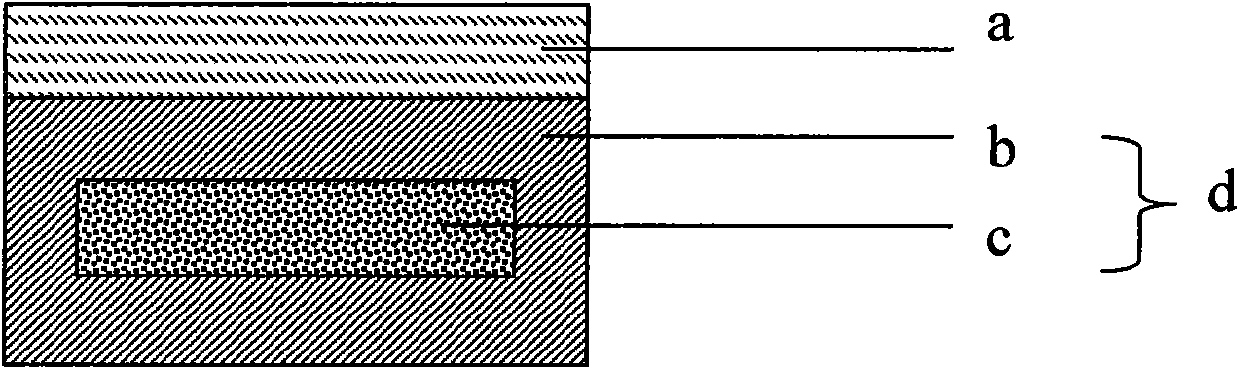
Musk Baoxin long-acting medicine product and preparation method thereof
A musk heart-protecting and drug product technology, which is applied in the direction of medical formula, drug combination, drug delivery, etc., can solve the problems of volume and ratio changes, pharmacological effects and curative effect reduction, etc., achieve small dosage, reduce myocardial infarction area, improve left The effect of chamber remodeling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
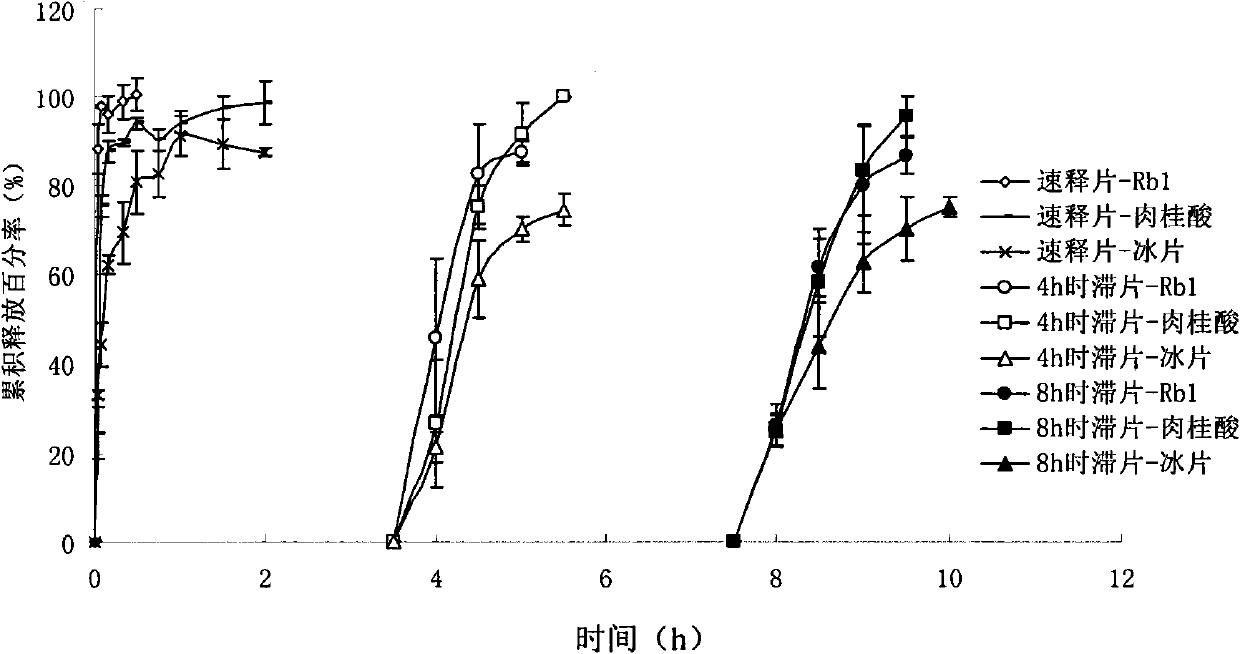
[0041] Preparation of immediate-release tablets: 20% bulk drug (provided by Shanghai Hutchison Pharmaceutical Co., Ltd.) with 10% disintegrating agent crospovidone, 10% disintegrating agent sodium carboxymethyl starch, 59% filler microcrystalline cellulose Su and 1% lubricant micro-powder silica gel are mixed in proportion, and the powder is directly compressed into tablets to obtain the product. Each tablet weighs about 78mg. Dissolution test results showed that ginsenoside Rb 1 , cinnamic acid and borneol 45min cumulative dissolution percentage> 80%.
[0042] Preparation of pulse-release tablets:
[0043] ① Tablet core: mix 60% raw material drug (provided by Shanghai Hutchison Pharmaceutical Co., Ltd.) with 20% disintegrant crospovidone, and 20% filler microcrystalline cellulose, and use a 4mm die to compress the powder directly. That is to get the tablet core, each tablet weighs about 26mg. Dissolution test results showed that ginsenoside Rb 1 , cinnamic acid and borne...
Embodiment 2
[0048] The preparation of immediate-release tablet: 20% crude drug (provided by Shanghai Hehuang Pharmaceutical Co., Ltd.) is mixed with 7% disintegrant crospovidone, 72% filler microcrystalline cellulose, 1% lubricant micropowder silica gel in proportion Evenly, the powder is directly compressed into tablets. Each tablet weighs about 78mg. Dissolution test results showed that ginsenoside Rb 1 , cinnamic acid and borneol 45min cumulative dissolution percentage> 80%.
[0049] Preparation of pulse-release tablets:
[0050] ① Tablet core: Mix 60% raw material drug (provided by Shanghai Hutchison Pharmaceutical Co., Ltd.) with 5% disintegrating agent crospovidone and 35% filler lactose, and use a 4mm die to compress the powder directly to obtain tablets Core, each tablet weighs about 26mg.
[0051] ②Short-time-delay pulse-release tablet: the coating material 39mg HPMC E15 (accounting for 60% of the pulse-release layer) by the second compression method, floating layer material:...
Embodiment 3
[0055] The preparation of immediate-release tablet: 20% bulk drug (provided by Shanghai Hehuang Pharmaceutical Co., Ltd.) and 3% disintegrant sodium carboxymethyl cellulose, 76% filler compressible starch, 1% lubricant micropowder silica gel in proportion Mix well, and the powder is directly compressed into tablets to obtain the product. Each tablet weighs about 78mg. Dissolution test results showed that ginsenoside Rb 1 , cinnamic acid and borneol 45min cumulative dissolution percentage> 75%.
[0056] Preparation of pulse-release tablets:
[0057] ① Tablet core: mix 60% raw material medicine (provided by Shanghai Hutchison Pharmaceutical Co., Ltd.) with 10% disintegrant sodium carboxymethyl starch and 30% compressible starch, use a 4mm die, and directly compress the powder into tablets to obtain Tablet core, each tablet weighs about 26mg.
[0058] ②Short-time-delay pulse-release tablet: use the second compression method to coat material 105mg HPMC E15 (80% of the pulse-re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com