1-aryl-3-substituted-5-substituted amino-4-pyrazolecarboxamide compounds and their applications
A pyrazole carboxamide and compound technology, applied in the field of medicine, can solve the problems of poor selectivity, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the synthesis of compound (1)
[0033]
[0034] Adopt the mode of synthetic route I:
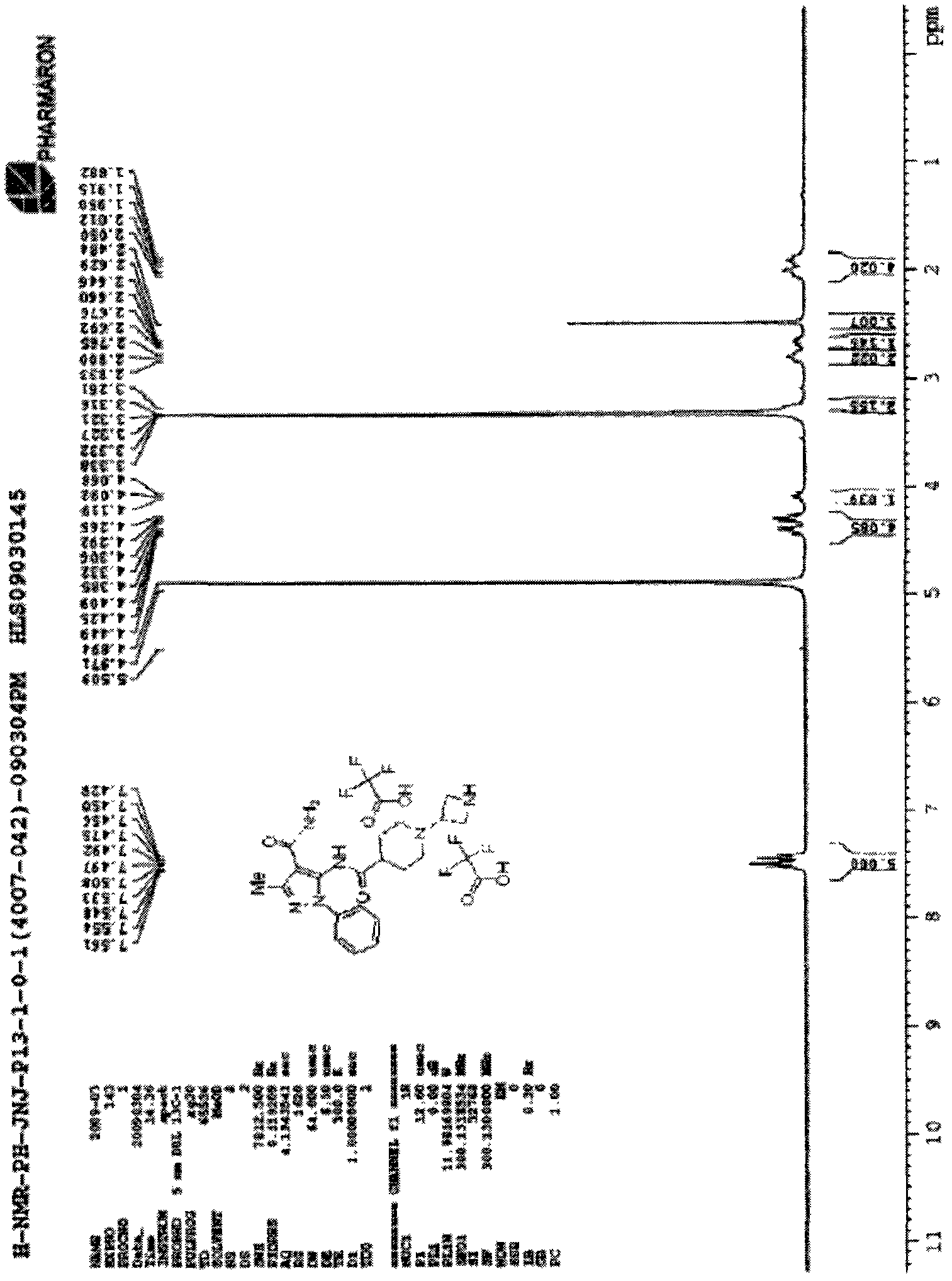
[0035] 2-Cyanoacetamide (3.36 g, 39.96 mmol) was dissolved in a 250 mL round bottom flask with 7 mL of acetic acid, and 1,1,1-triethoxyethane (7.13 g, 43.95 mmol) was added to the flask , the whole reaction system was stirred overnight at 100° C., and after the reaction was completed, it was lowered to room temperature. Add 15 mL of diethyl ether to the reaction system, and at the same time collect the resulting solid by filtration and dry to obtain 3.3 g of white solid 2-cyano-3-ethoxybutyl-2-enamine, melting point: 95-97°C, yield 54 %, (ES, m / z): 155.2 [M+H] +1 .
[0036] 2-Cyano-3-ethoxybutyl-2-enamine (1.0 g, 6.49 mmol) was dissolved in 15 mL of methanol in a 50 mL round bottom flask while phenylhydrazine (840 mg, 7.77 mmol) was added. The whole reaction system was stirred overnight at 80° C., and the reaction was completed. The reaction solution was concentr...
Embodiment 2
[0038] Embodiment 2: Using 1-pyridine-4-piperidine-4-formyl chloride as a reagent, according to the synthetic route I, compound (2) can be prepared
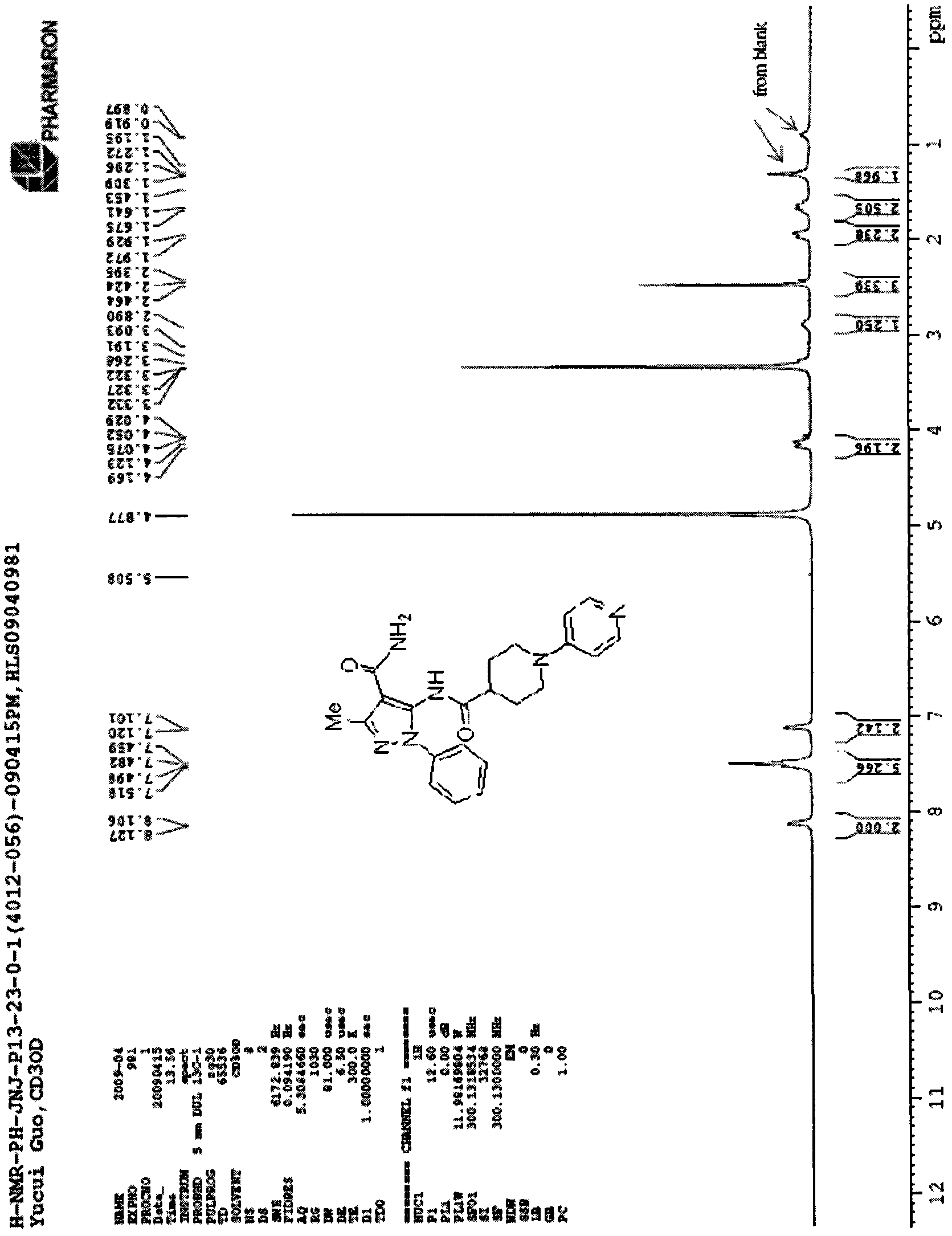
[0039]
[0040] Mp (melting point): 101~103℃; (ES, m / z): 421.2[M+H] +1 ; 1 H NMR (300MHz, DMSO) δ: 1.30(s, 2H), 1.64~1.68(m, 2H), 1.93~1.97(m, 2H), 2.46(s, 3H), 2.89(brs, 1H), 4.02( s, 3H), 4.15 (d, 2H), 7.10-7.12 (m, 2H), 7.45-7.52 (m, 5H), 8.10-8.13 (m, 2H). See figure 2 .
Embodiment 3
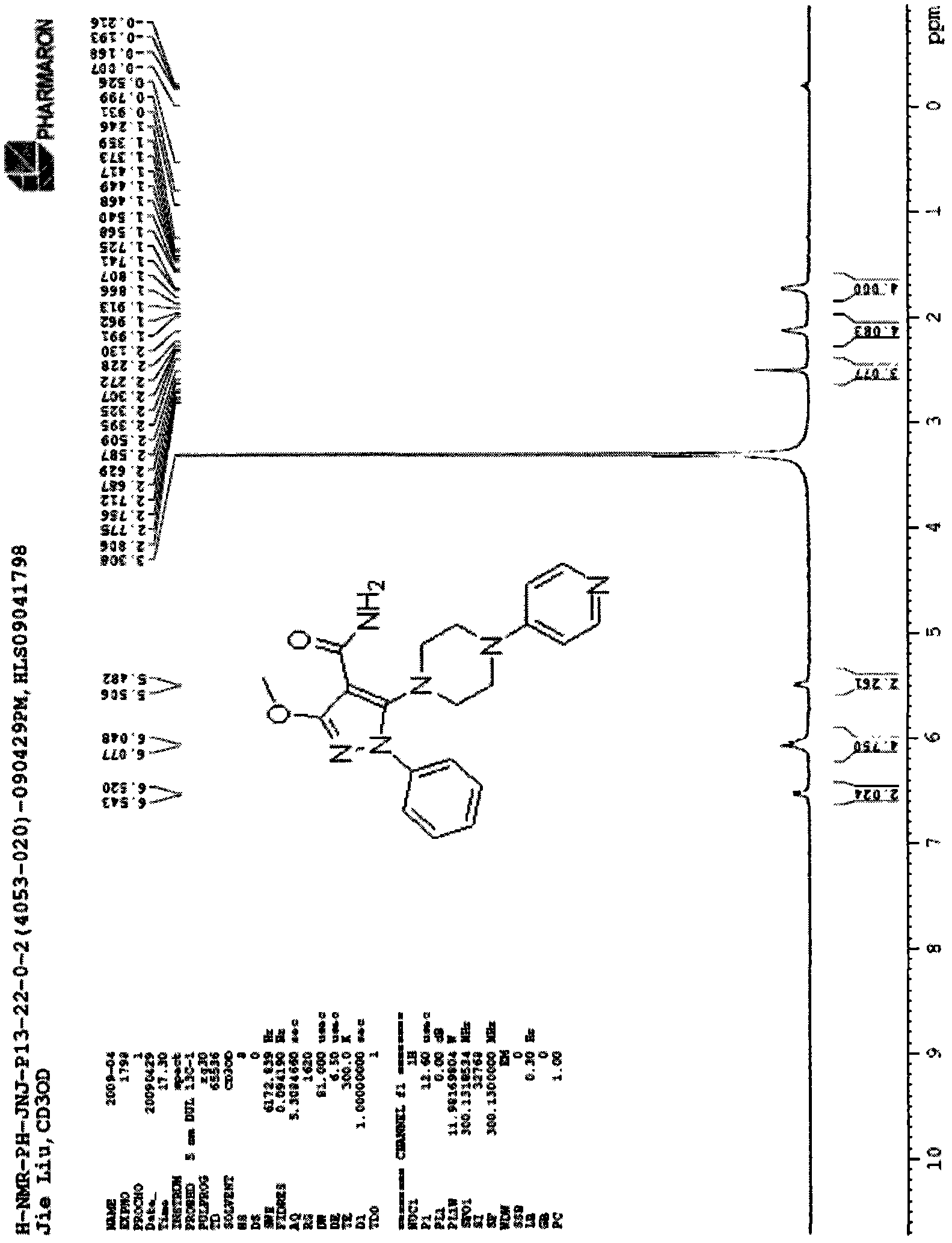
[0041] Embodiment 3: Using 1-(azetidine-3)-piperidine-4-formyl chloride as a reagent, according to the synthetic route I, the synthesis of compound (3) can be obtained
[0042]
[0043] Mp (melting point): 121~123℃; (ES, m / z): 383.2[M+H] +1 ; 1 H NMR (300MHz, DMSO) δ: 1.88~2.05(4H, m), 2.48(3H, s), 2.63~2.69(1H, m), 2.80(2H, t), 3.20~3.30(2H, m), 4.06-4.12 (1H, m), 4.26-4.45 (4H, m), 7.42-7.56 (5H, m). See image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com