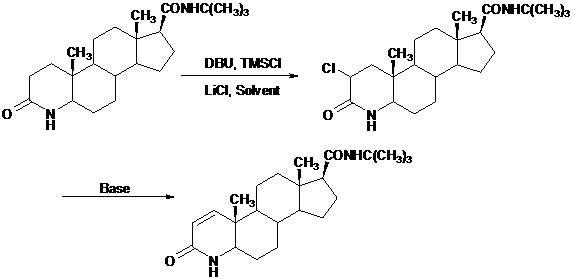
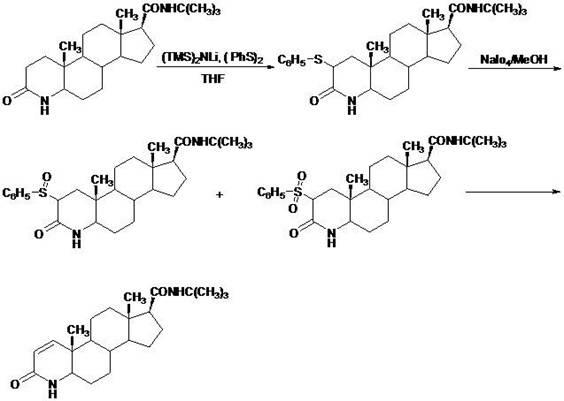
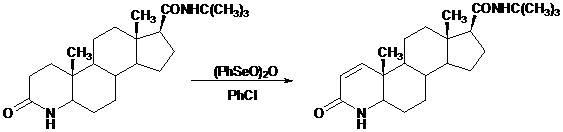Novel technology for synthesis of finasteride via chlorination and dehydrogenation
A technology of dihydrofinasteride and finasteride, which is applied in the directions of steroids and organic chemistry, can solve the problems of high price, complicated operation and high cost, and achieves fast reaction speed, simple operation and less waste pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In a 1000 ml three-neck flask, under nitrogen protection, add 50.0 g of dihydrofinasteride, then add 500 ml of 1,4-dioxane, stir to dissolve the solid, cool to -5°C, and slowly add 24 ml DBU (1,8-diazabicyclo[5,4,0]undec-7-ene), keep the temperature at -5℃~0℃ during the addition process, then add 35ml in 1~2 hours After the addition of trimethylchlorosilane, stir and react at 25°C for 2 hours, then add 12.0 grams of anhydrous lithium chloride at one time, raise the temperature to reflux temperature, and react completely at this temperature. The solvent was distilled off under pressure, 200 ml of ethanol was added to the residue, fully stirred and heated to dissolve, and after cooling, it was slowly poured into an aqueous ammonium chloride solution to precipitate the product, and the crude 2-chloro-dihydrophena was obtained after filtering, washing, and other steps For the androstamine product, the crude product was dried under reduced pressure at 40°C to 70°C; 51.6 ...
Embodiment 2
[0034] In a 1000 ml three-necked flask, under nitrogen protection, add 50.0 g of dihydrofinasteride, then add 300 ml of DMF (N,N-dimethylformamide), stir to dissolve the solid, cool to -5°C, Slowly add 26 ml of DBU (1,8-diazabicyclo[5,4,0]undec-7-ene), keeping the temperature at -5°C~0°C during the addition, and then at 1~2 Add 35 milliliters of trimethylchlorosilane within 1 hour. After the addition, stir and react at 25°C for 2 hours, then add 11.4 grams of anhydrous lithium chloride at one time, and raise the temperature to the reflux temperature. The reaction is complete, the solvent is distilled off under reduced pressure, 200 ml of ethanol is added to the residue, fully stirred and heated to dissolve, after cooling, it is slowly poured into an aqueous solution of ammonium chloride to precipitate the product, and the crude 2-chloro- Dihydrofinasteride product, the crude product was dried under reduced pressure at 40°C~70°C; 52.3 grams of light yellow powdery solid 2-c...
Embodiment 3
[0037] In a 1000 ml three-neck flask, under nitrogen protection, add 50.0 g of dihydrofinasteride, then add 500 ml of tetrahydrofuran, stir to dissolve the solid, cool to -5°C, and slowly add 1M n-butyllithium hexane 147 ml of solution, keep the temperature at -78°C during the addition process, then react at this temperature for 1 hour, slowly add 35 ml of trimethylchlorosilane dropwise, maintain the reaction at this temperature for 2 hours, make the temperature slowly Raise to 25°C, add 13.0 g of anhydrous lithium chloride at one time, raise the temperature to reflux temperature, and the reaction is complete at this temperature, carefully add 20 ml of water to quench the reaction, distill the solvent under reduced pressure, and add 200 milliliters of ethanol, fully stirred and heated to dissolve, slowly poured into the ammonium chloride aqueous solution to precipitate the product after cooling, obtained the crude 2-chloro-dihydrofinasteride product through steps such as fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com