Method for detecting trace trivalent arsenic through two-signal anodic stripping voltammetry
An anode stripping voltammetry and trivalent arsenic technology, which is applied in the field of electrochemistry, can solve the problem of large errors, no high-sweep-speed linear sweep voltammetry and high-sweep-rate cyclic voltammetry, and the accuracy of detection results is not high, etc. problems, to achieve the effect of convenient operation, satisfactory detection results, and enhanced detection flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
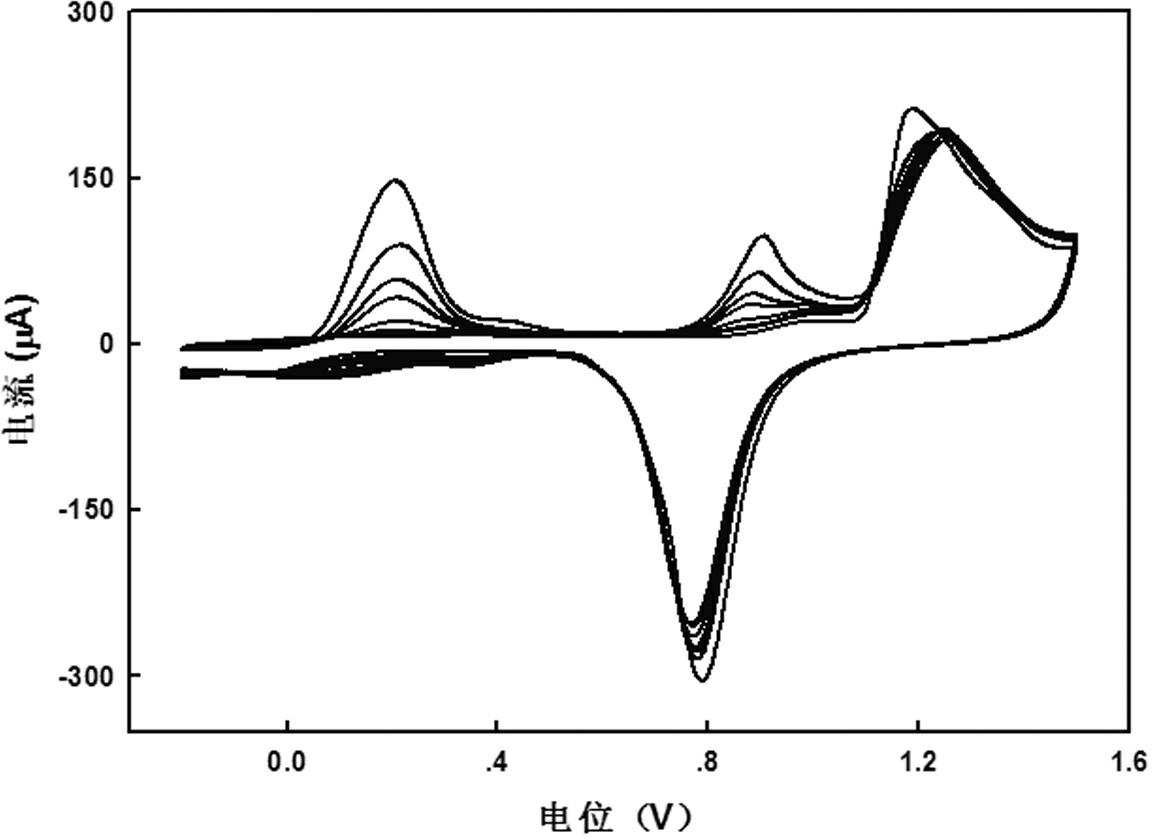
[0021] Example 1: High-sweep cyclic voltammetry detection laboratory As (Ⅲ) standard sample
[0022] Standard sample preparation: prepare 0.1 mol L -1 Trivalent arsenic, dilute this solution to 0.01, 0.1, 1 and 10 mmol·L when using -1 . Prepare 0.1 mol L with ultrapure water -1 sulfuric acid solution.
[0023] Sample determination: detection by dual-signal anodic stripping voltammetry. (1) Using a three-electrode system, thoroughly clean the gold electrode used as the working electrode, the saturated calomel electrode used as the reference electrode, the platinum disk electrode used as the counter electrode, the plastic beaker, and the magnetic stirrer with water. (2) Connect the line, fix the position of the beaker, electrodes and magnetic stirrer, add 10 mL of 0.1 mol·L -1 Sulfuric acid, for electrochemical studies. (3) Using cyclic voltammetry, a blank test was performed first, and then a certain concentration of standard As(Ⅲ) solution was added to the solution to en...
Embodiment 2
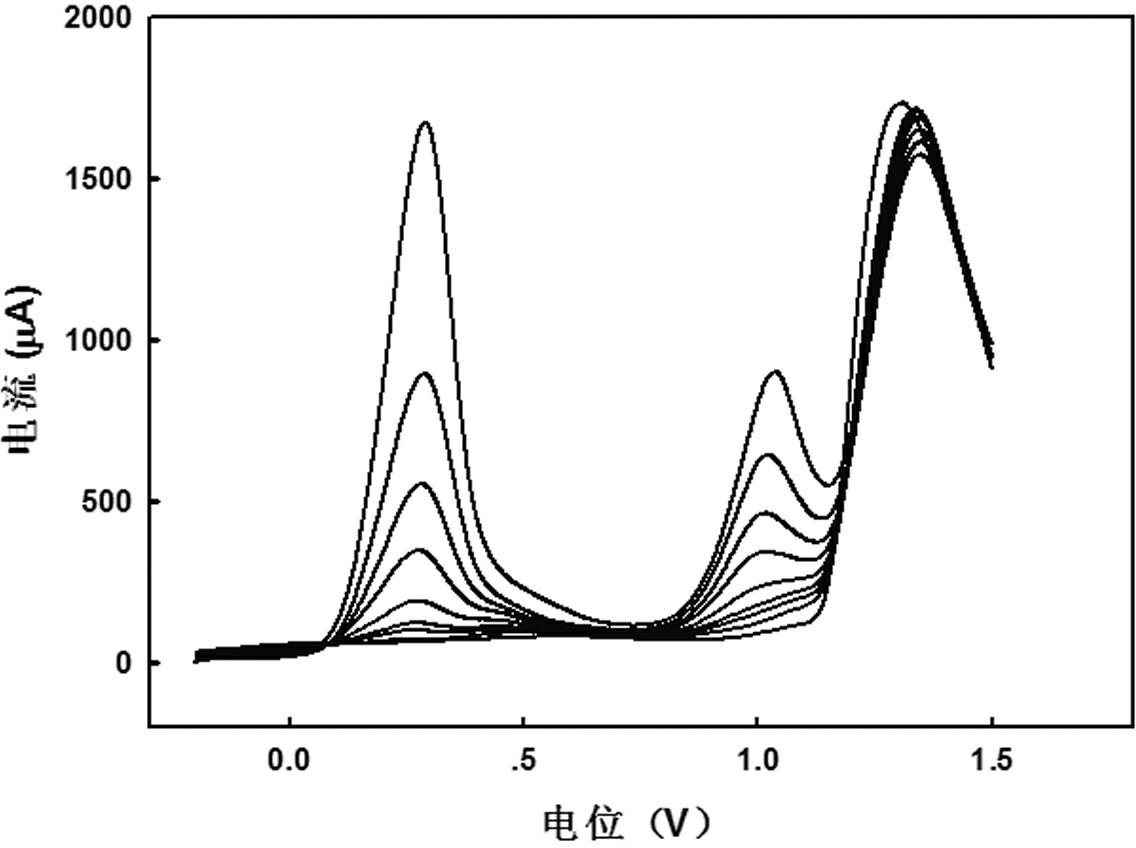
[0025] Embodiment 2: High-scan rate linear sweep voltammetry detection laboratory As(Ⅲ) standard sample
[0026] Standard sample preparation: similar to Example 1. The difference is that 0.5 mol L was prepared with ultrapure water -1 Sulfuric acid solution was detected by high-speed linear sweep voltammetry.
[0027] Sample determination: detection by dual-signal anodic stripping voltammetry. Step 1 is similar to Example 1; add 10 mL of 0.5 mol L in step 2 -1 Sulfuric acid, for electrochemical studies. (3) Using high-speed linear sweep voltammetry, first conduct a blank test, then add a certain concentration of standard As(Ⅲ) solution to the solution, and enrich for 400 s at -0.2 V vs SCE potential (the first 390 s solution stirring, and the solution was left to stand for the last 10 s), and then scanned forward to 1.5 V at a scan rate of 10 V / s, so that the enriched As(0) was electrooxidized and stripped into As(Ⅲ), As(Ⅲ) for further electro-oxidation Oxidize to As(Ⅴ), r...
Embodiment 3
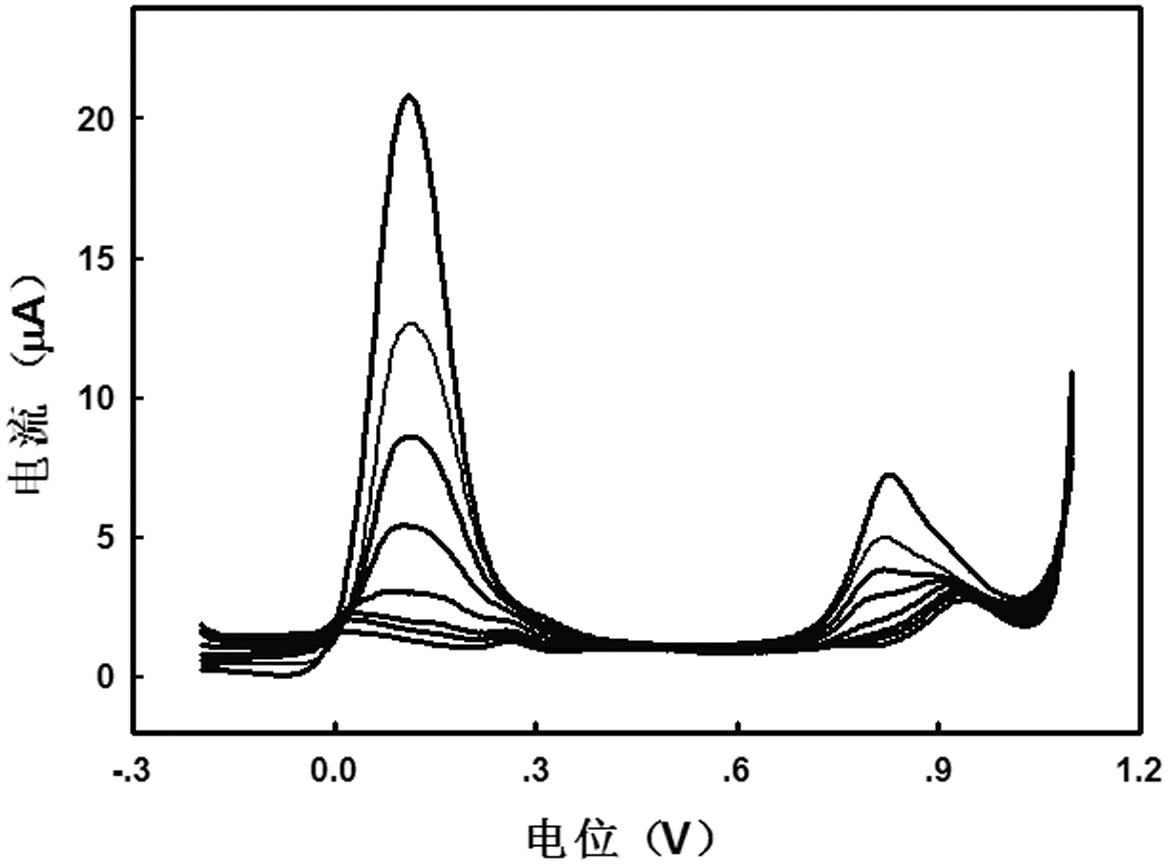
[0029] Embodiment 3: square wave voltammetry detection laboratory As (Ⅲ) standard sample
[0030] Standard sample preparation: similar to Example 1, using ultrapure water to prepare 0.5 mol L -1 Sulfuric acid solution is detected by square wave voltammetry.
[0031]Sample determination: detection by dual-signal anodic stripping voltammetry. Steps 1 and 2 are similar to those in Example 1; (3) Using square wave voltammetry, first conduct a blank test, and then add a certain concentration of standard As(Ⅲ) solution to the solution to enrich the concentration at -0.2 V vs SCE potential Set for 400 s (the solution was stirred for the first 390 s, and the solution was left standing for the last 10 s), and then scanned forward to 1.1 V to electrooxidize the enriched As(0) into As(Ⅲ) and As(Ⅲ) for further electrooxidation into As(Ⅴ), record the double response signal of oxidation current.
[0032] Analysis of assay results: combined image 3 For discussion, the oxidation peak pot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com