Metoprolol slow-release microsphere, slow-release medical composition and preparation method of metoprolol slow-release microsphere
A slow-release microsphere and microsphere technology, applied in the field of medicine, can solve the problems of complex prescription process, poor controllability, short sustained-release time, etc., and achieve the effects of strong controllability, low cost and high encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
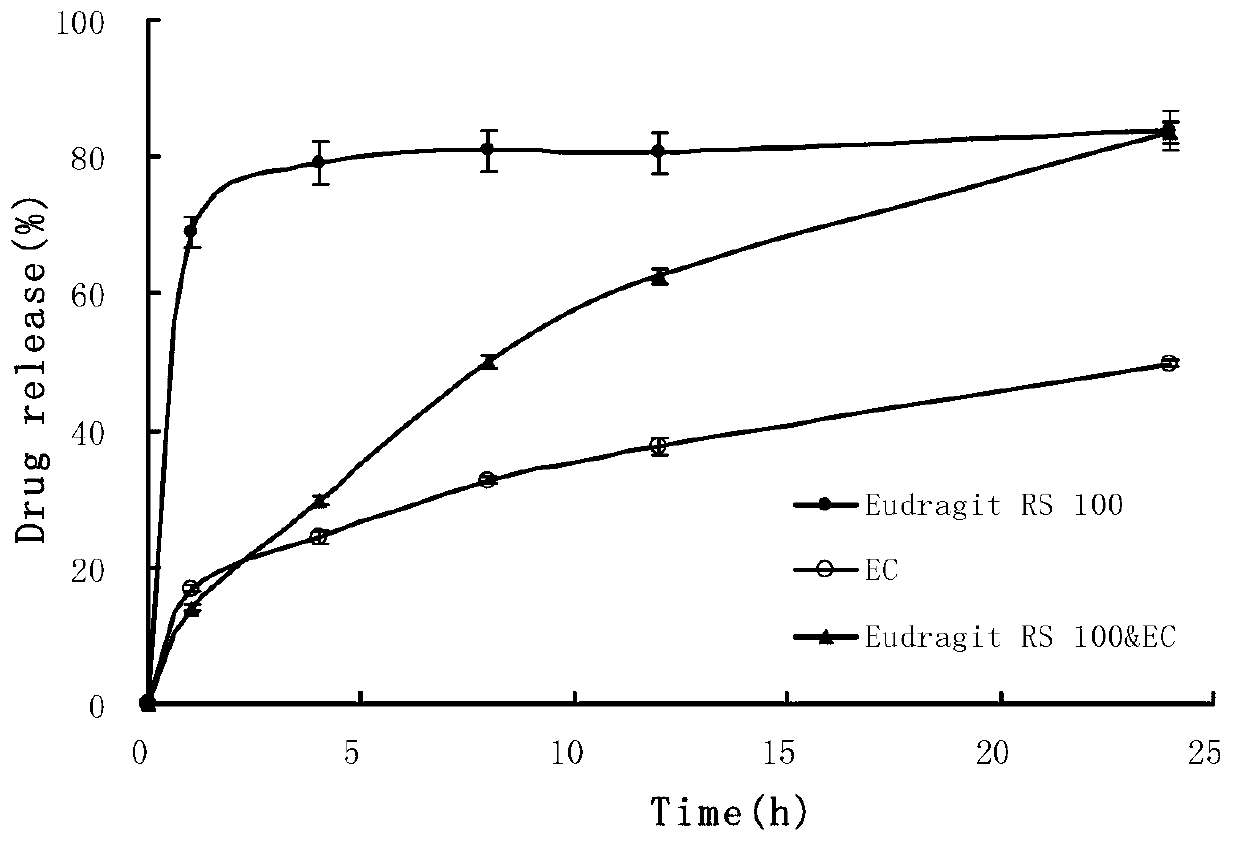
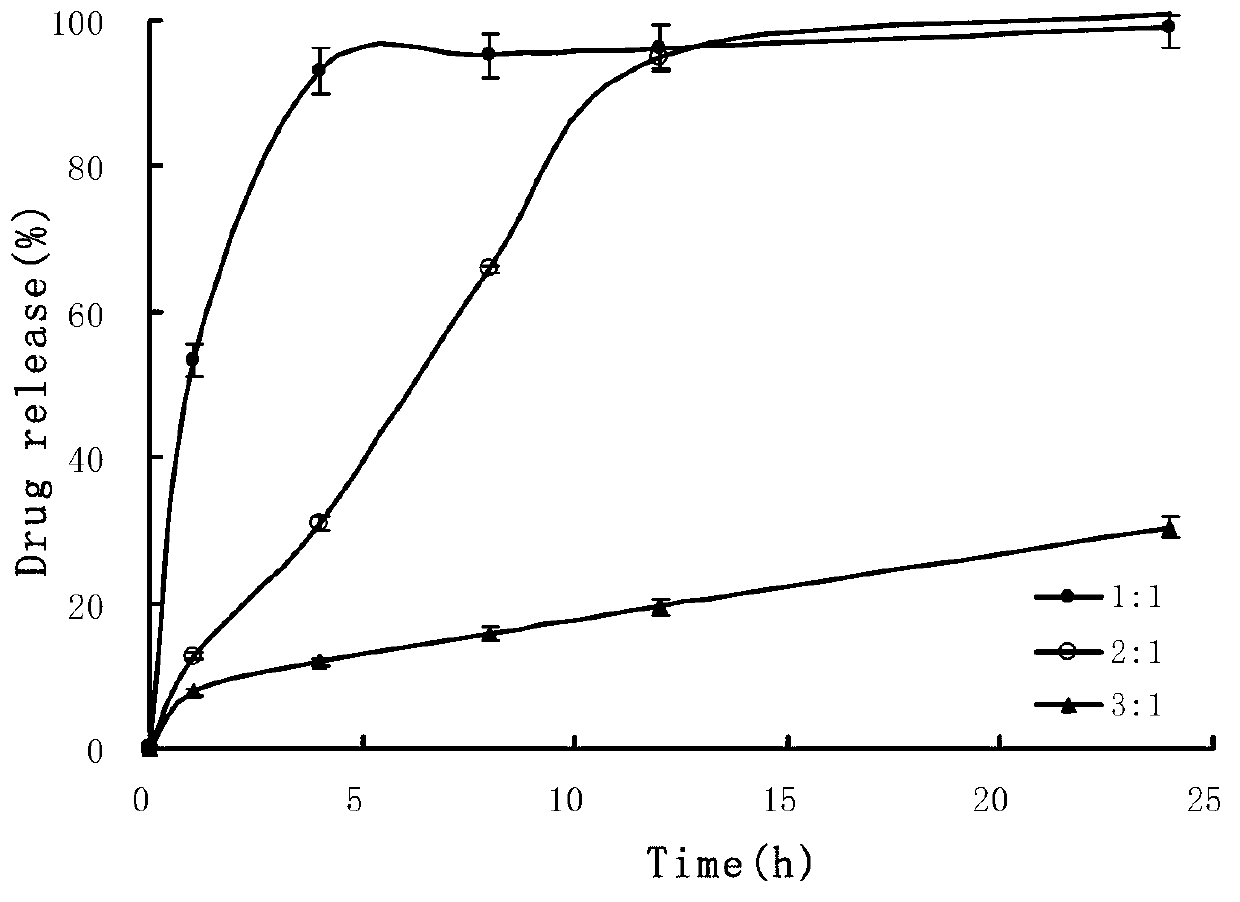
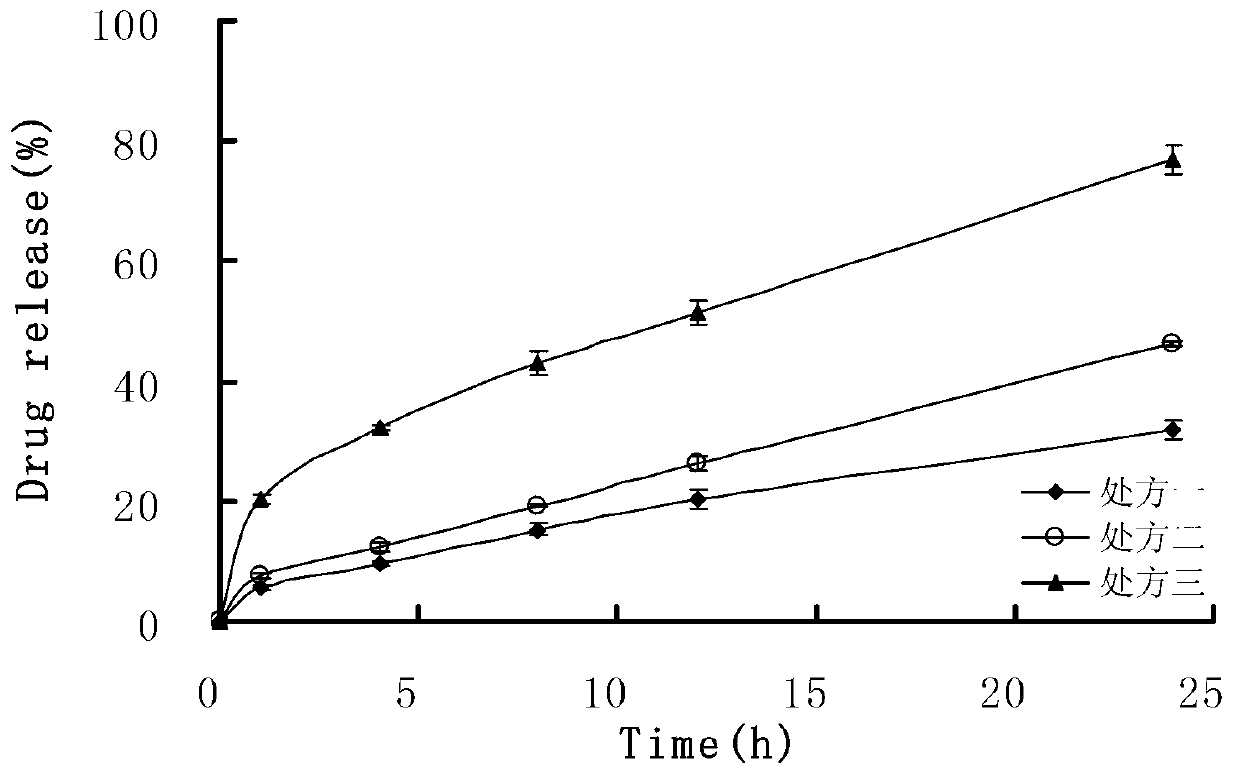
[0055] The metoprolol sustained-release microspheres of the present embodiment are prepared from 40wt% of the pharmaceutical active ingredient and 60wt% of the sustained-release material, the pharmaceutical active ingredient is metoprolol hydrochloride, and the sustained-release material 65 wt% ethyl cellulose with 35 wt% Eudragit RS 100. The specific composition of the slow-release microspheres is: 4g of metoprolol hydrochloride, 3.9g of ethyl cellulose and 2.1g of Eudragit RS 100. Sustained-release microspheres and excipients are mixed with a mass ratio of 25: 1 to prepare a pharmaceutical composition. The excipients are 0.2 g each of magnesium stearate and silicon dioxide. The specific preparation method includes the following steps:
[0056] (1) prepare organic solvent, described organic solvent is the mixed solution of ethyl acetate: ethanol=8: 2 (v / v);
[0057] (2) Add the medicinal active ingredient and sustained-release material in proportion to the mixed solution of ...
Embodiment 2
[0062] The metoprolol sustained-release microspheres of the present embodiment comprise 8wt% of pharmaceutically active ingredients and 92wt% of sustained-release materials, wherein the pharmaceutically active ingredients are metoprolol succinate, and the sustained-release materials It is 75 wt% ethyl cellulose and 25 wt% polyaminomethacrylate. The specific composition of the metoprolol sustained-release microspheres is: 0.8 g of metoprolol succinate, 6.9 g of ethyl cellulose and 2.3 g of Eudragit RL 100 as sustained-release materials. Metoprolol sustained-release microspheres and excipients prepare the metoprolol sustained-release microspheres pharmaceutical composition with a mass ratio of 50:1, wherein the excipients are magnesium stearate, talcum powder, 0.1g, the specific preparation method comprises the following steps:
[0063] (1) prepare organic solvent, described organic solvent is the mixed solution of chloroform: ethanol=8: 2 (v / v);
[0064] (2) Add the medicinal...
Embodiment 3
[0069] The metoprolol sustained-release microspheres of the present embodiment comprise 20wt% of the pharmaceutically active ingredient and 80wt% of the sustained-release material, wherein the pharmaceutically active ingredient is metoprolol tartrate, and the sustained-release material is 71 wt% ethyl cellulose and 29 wt% polyaminomethacrylate. The specific composition of the microspheres is: 2g of metoprolol tartrate and 5.68g of ethylcellulose and 2.32g of Eudragit RS100. The release microspheres and excipients are mixed in a mass ratio of 10:1 to prepare a pharmaceutical composition containing sustained release microspheres. The excipients are 0.8 g of BASF, magnesium stearate, and silicon dioxide. 0.1g, the specific preparation method comprises the following steps:
[0070] (1) prepare organic solvent, described organic solvent is 80% (v / v) ethanol solution;
[0071] (2) Add the medicinal active ingredient and sustained-release material in proportion to the solution obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com