Local used composition containing analgesic, and preparation method and use of local used composition
A technology for topical use and composition, applied in the field of pharmacy, can solve the problems of easy decomposition of ozone, limited application, low solubility, etc., and achieve the effects of easy portability, reduced toxicity and addiction, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
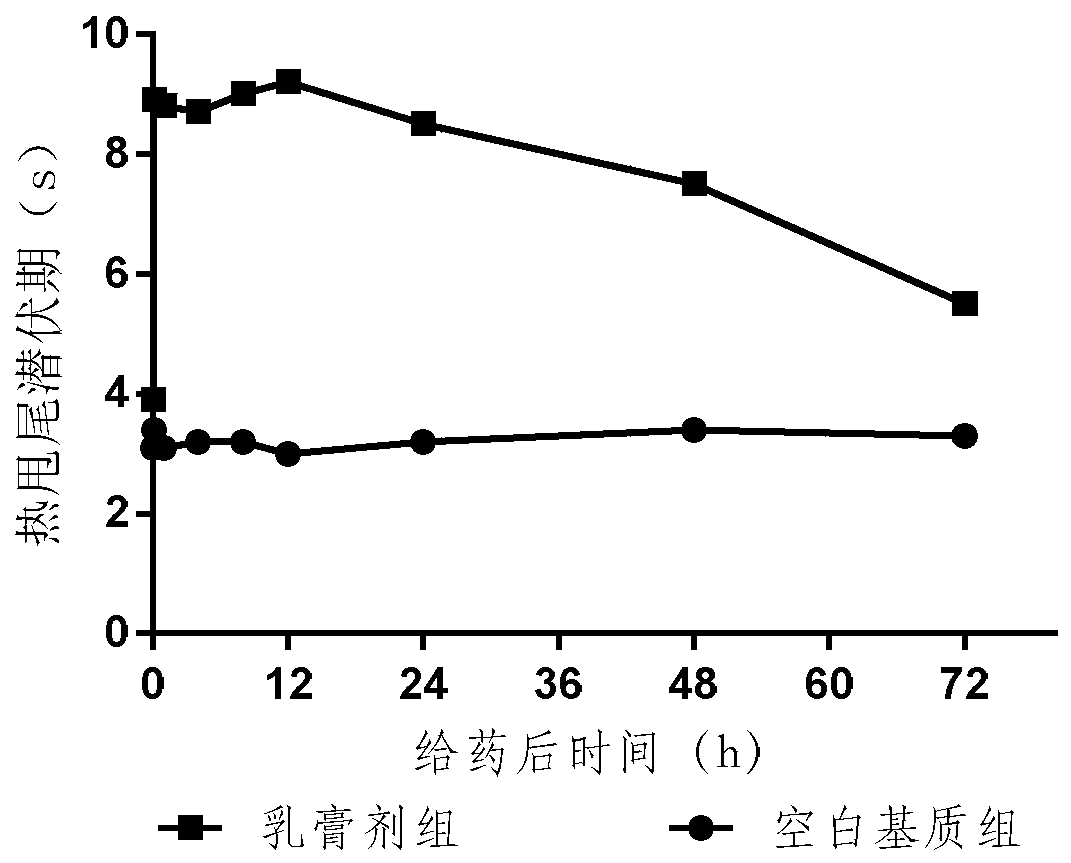
Embodiment example 1
[0061] Prepare the prescription as follows: 10mg / 100g, the paste is calculated as 100g:
[0062]
[0063]
[0064] Preparation Process:
[0065] (1) Dissolve β-cyclodextrin and hydromorphone hydrochloride in water, control the pH at 6.0 to 7.5 by adding a buffer solution, and fully include β-cyclodextrin and the drug through ultrasound. The temperature is between 20 and 30°C.
[0066] (2) Add sodium sulfite to the aqueous solution of β-cyclodextrin inclusion drug.
[0067] (3) Dissolve the egg yolk lecithin and olive oil in the organic solvent ethyl acetate under stirring to obtain the oil phase, and pass through the prescription amount of ozone, add the cream base and transdermal absorbent, and put it in a water bath at 40-50°C Heating at medium temperature for 30-60 minutes;
[0068] (4) The oil phase is added to the water phase under stirring conditions, and an emulsion is prepared by using a high-speed shearing instrument, and the particle size is controlled below...
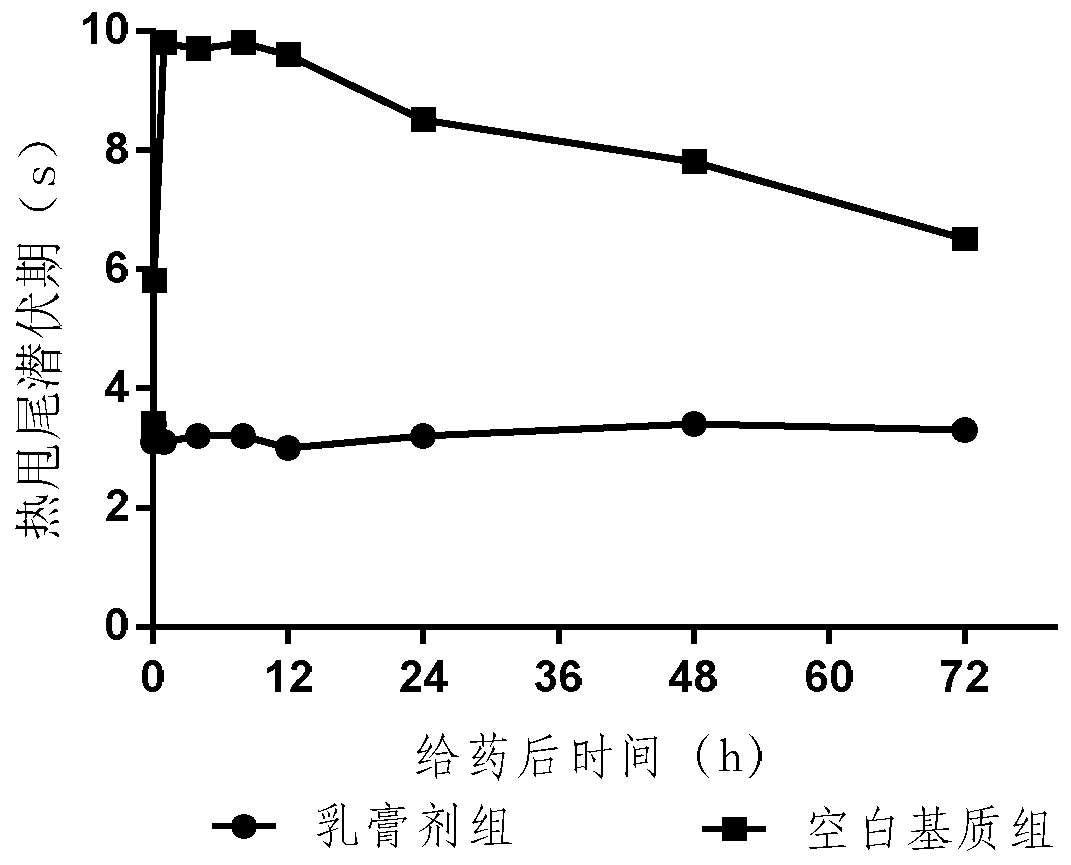
Embodiment example 2
[0073] The preparation prescription is as follows, the specification is 5mg / 100g, and the paste is calculated as 100g:
[0074]
[0075] Preparation Process:
[0076] (1) Dissolve hydroxypropyl-β-cyclodextrin and nalbuphine hydrochloride in water, control the pH at 6.0-7.5 by adding a buffer solution, and fully encapsulate the hydroxypropyl-β-cyclodextrin and the drug by ultrasonic In the process of ultrasonic inclusion, the temperature is controlled at 20-30°C.
[0077] (2) Add sodium thiosulfate to the aqueous solution of hydroxypropyl-β-cyclodextrin inclusion drug.
[0078] (3) Dissolve the poloxamer and olive oil in the organic solvent ethyl acetate under stirring to obtain the oil phase, and inject the prescribed amount of ozone, add the cream base and the transdermal absorbent, and heat the mixture at 40-50°C Heating in a water bath for 30-60 minutes;
[0079] (4) The oil phase is added to the water phase under stirring conditions, and an emulsion is prepared by us...
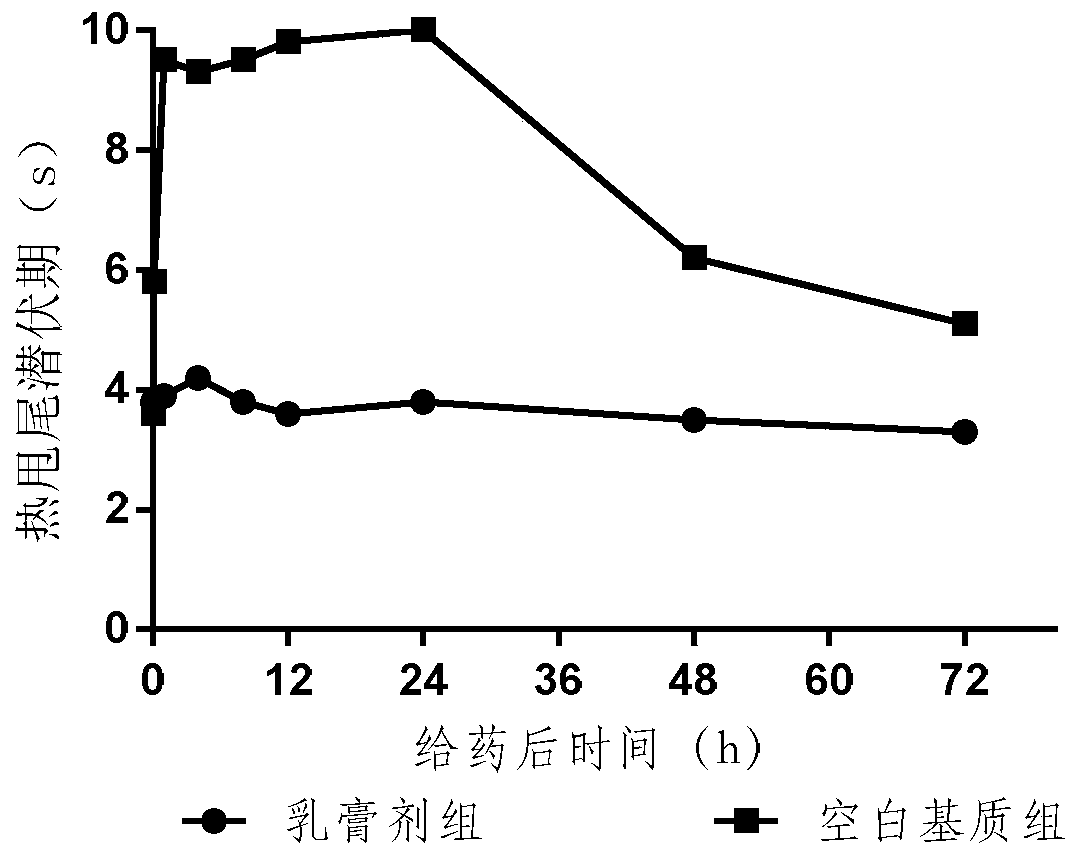
Embodiment example 3
[0084] Prepare the prescription as follows, the specification is 0.1mg / 100g, and the paste is calculated as 100g:
[0085]
[0086] Preparation Process:
[0087] (1) Dissolve hydroxyethyl-β-cyclodextrin and sufentanil citrate in water, control the pH at 6.0-7.5 by adding buffer solution, and make hydroxyethyl-β-cyclodextrin and The drug is fully included, and the temperature is controlled at 20-30°C during the ultrasonic inclusion process.
[0088] (2) Add propyl gallate to the aqueous solution of hydroxyethyl-β-cyclodextrin inclusion drug.
[0089] (3) Dissolve the egg yolk lecithin and olive oil in the organic solvent ethyl acetate under stirring to obtain the oil phase, and pass through the prescription amount of ozone, add the cream base and transdermal absorbent, and put it in a water bath at 40-50°C Heating at medium temperature for 30-60 minutes;
[0090] (4) Adding the oil phase to the water phase under stirring conditions, using a high-speed shearing instrument ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com