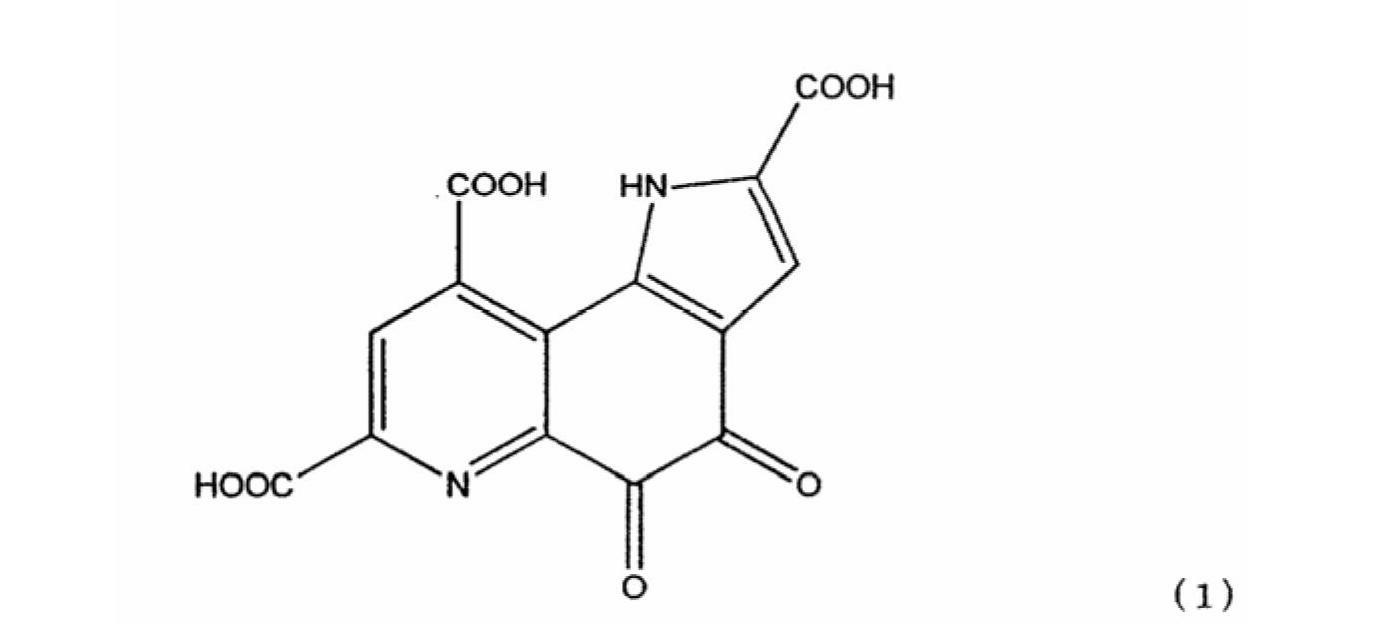
Pyrroloquinoline quinone in free form
A compound and manufacturing method technology, applied in the direction of organic chemistry, can solve the problems of poor solubility, inefficiency, and cost of PQQ, and achieve the effect of good crystallinity and low alkali metal content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] A reagent (trade name: BioPQQ) of MITSUBISHI GAS CHEMICAL COMPANY, INC. was used as a raw material PQQ disodium salt. The purity of PQQ disodium salt was 99.0% as determined by UV absorption on high performance liquid chromatography.
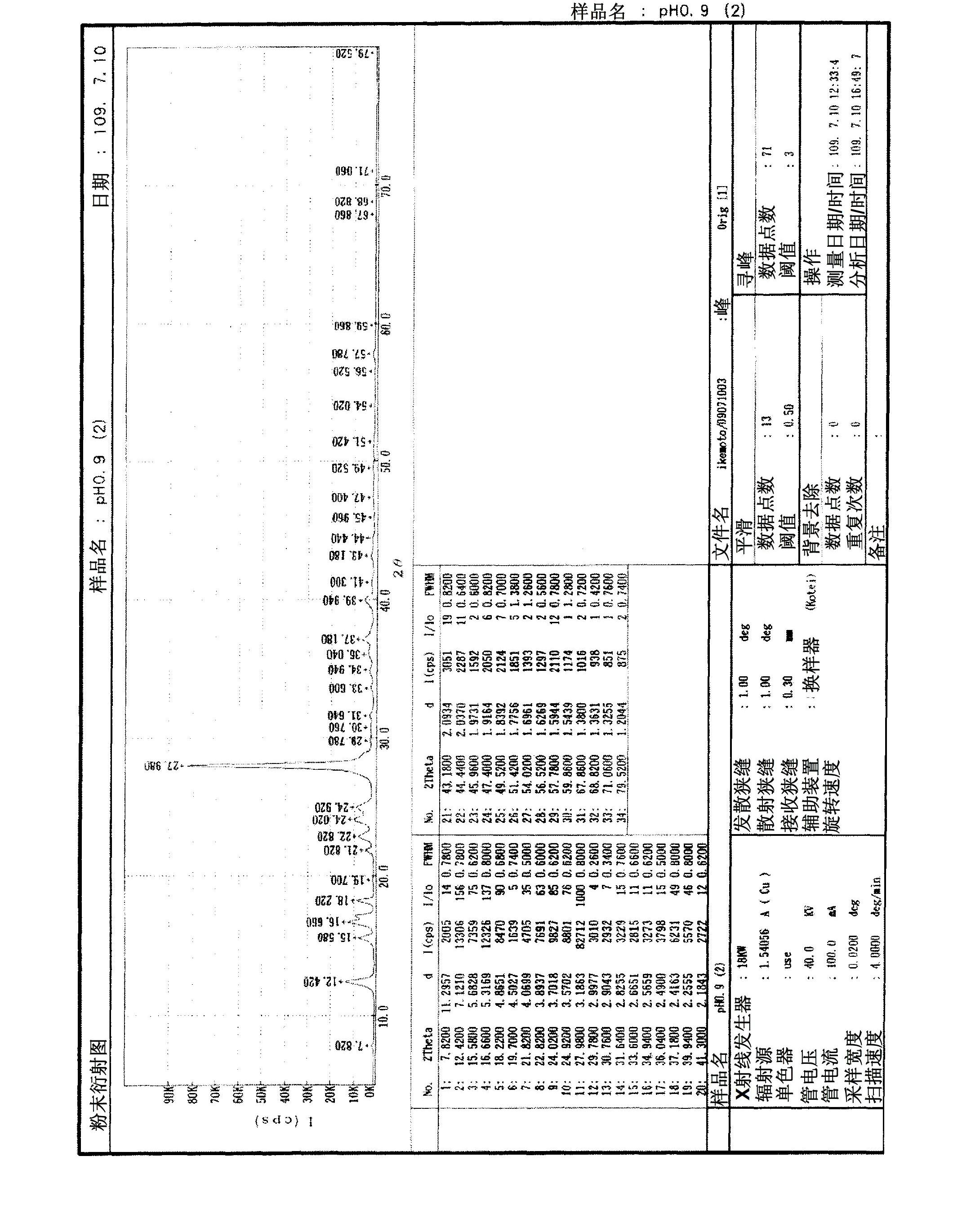
[0118] 2 g of the above disodium salt were added to 198 g of water to obtain an aqueous disodium salt solution. NaOH was added to the resulting solution to adjust the pH to 9. To this solution, 7.7 g of a solution obtained by diluting concentrated hydrochloric acid (from Wako Pure Chemical Industries, Ltd.) by 50% with water was added to adjust the pH to 0.9. After stirring for 30 minutes, the precipitated solid was filtered, washed with water and isopropanol. The material was dried overnight at 50°C under reduced pressure. The collected red crystals weighed 1.6 g. Na analysis showed that PQQ in free form with a Na content of 0 and thus no sodium was obtained by this simple method. The powder X-ray diffraction result of the free form...
Embodiment 2
[0120] The raw material (PQQ disodium salt) in Example 1 was dissolved in water. Sodium hydroxide was added to the solution to adjust the pH to 8, after which sodium chloride was added to precipitate PQQ trisodium salt. The precipitated PQQ trisodium salt was then washed with ethanol and dried. This salt was used in subsequent experiments.
[0121] 0.9 g of PQQ trisodium salt was dissolved in water (60 g). While stirring, about 2 g of concentrated hydrochloric acid was added thereto. The resulting solution had a pH of 0.6. After stirring overnight, the solution was filtered, the residue was washed with isopropanol and dried under reduced pressure to yield 0.35 g of a red solid. The results of powder X-ray diffraction and Na analysis of the resulting red solid were similar to those of Example 1, showing no residual Na in the red solid. It is shown as crystals of pyrroloquinoline quinone in free form.
Embodiment 3
[0123] To a mixed solution of 3.5 g of concentrated hydrochloric acid and 3.5 g of water was added 1 g of the same PQQ disodium salt solid as in Example 1 to adjust the pH of the solution to 1. After stirring at room temperature for 1 hour, the solution was filtered. The residue was washed with water and dried under reduced pressure to obtain 0.79 g of a red solid. The molar ratio of Na to PQQ in the resulting red solid was 0.06, indicating a small amount of sodium remained in the solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com