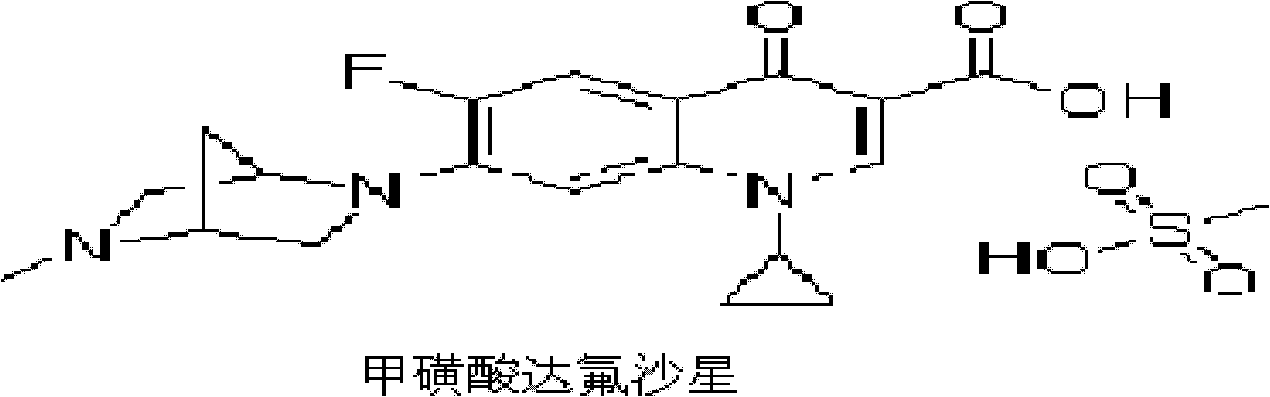
Danofloxacin mesylate suspension and preparation method thereof
A technology of danfloxacin mesylate and suspension, which is applied in the directions of liquid delivery, emulsion delivery, antibacterial drugs, etc. Tension, enhanced stability, delayed release effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
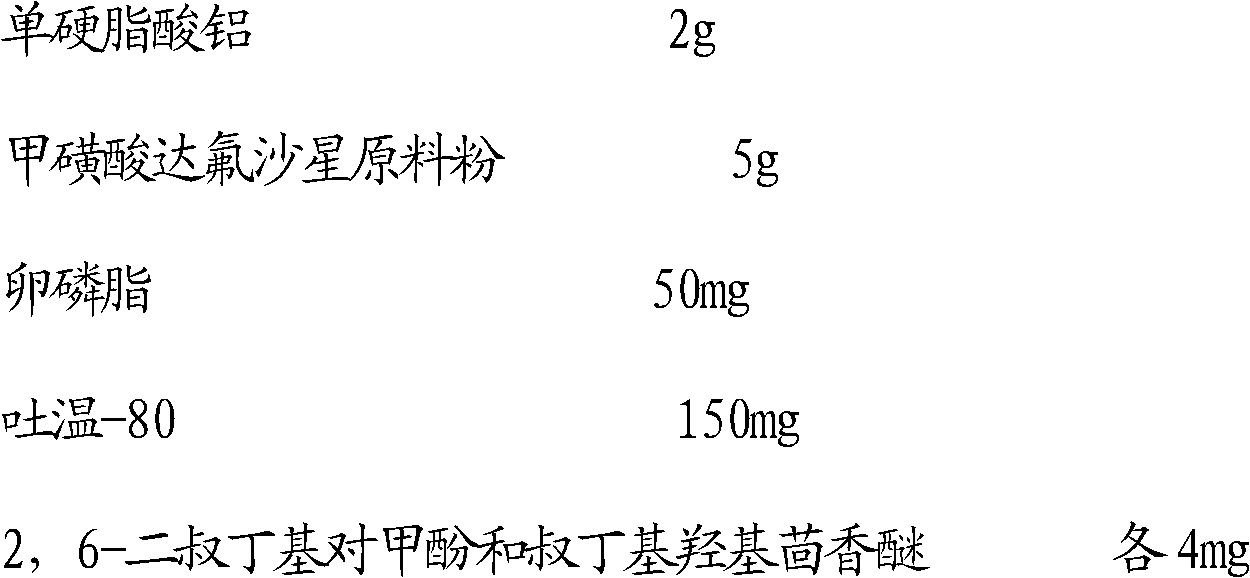
[0028] Embodiment 1: preparation 100ml mass volume ratio is 5% dafloxacin mesylate suspension
[0029] The actual dosage of the suspension is as follows:
[0030]
[0031] Preparation steps:
[0032] (1) Get 2g of aluminum monostearate, add it to 100ml of soybean oil for injection, stir while heating until the aluminum monostearate is completely dissolved, let stand and cool through No. 4 medicine sieve to obtain filtrate A;
[0033] (2) Put the dafloxacin mesylate raw material powder of the above quality in a beaker, add 50mg
[0034] Lecithin, 150mg Tween-80, 4mg antioxidant 2,6-di-tert-butyl-p-cresol, 4mg tert-butylhydroxyanisole and 10ml of the filtrate A obtained in the step were stirred evenly to obtain a mixture B;
[0035] (3) Place the mixture B obtained in step (2) in a disperser and add the remaining 90ml of filtrate A while dispersing; make the total volume to 100ml, wherein the dispersion speed is 8000r / min, and the dispersion time is 20min , to get mixture ...
Embodiment 2
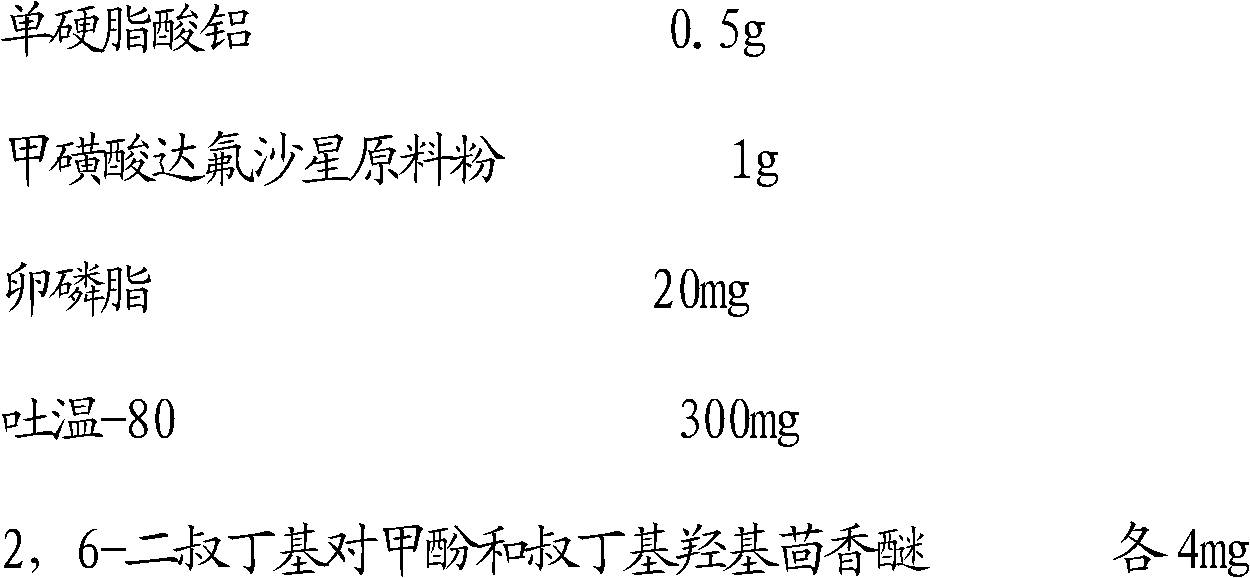
[0039] Embodiment 2: prepare 100ml 1% dafloxacin mesylate suspension
[0040] The actual dosage of the suspension is as follows:
[0041]
[0042] The suspension was prepared according to the preparation steps of Example 1. Wherein the filtrate A added in the step (2) is 20ml; The dispersion speed described in the step (3) is 13000r / min, and the dispersion time is 8min;
[0043] The quality inspection of the above suspension was found to be a milky yellow, slightly viscous suspension liquid, which will be layered after standing for a long time. The upper layer is a yellow liquid, and the lower layer is a milky white suspension. After vigorous shaking, it becomes uniform. milky yellow suspension, the pH of the liquid is 4.0-5.0. The relative labeled amount of dafloxacin mesylate is 99.46%, and the appearance and properties have no obvious change after high temperature and light exposure, and the content change is within 0.2%. The sedimentation volume ratio is more than 0....
Embodiment 3
[0044] Embodiment 3: prepare 100ml 2.5% dafloxacin mesylate suspension
[0045]
[0046]
[0047] The suspension was prepared according to the preparation steps of Example 1. The quality inspection of the above suspension was found to be a milky yellow, slightly viscous suspension liquid, which will be layered after standing for a long time. The upper layer is a yellow liquid, and the lower layer is a milky white suspension. After vigorous shaking, it becomes uniform. milky yellow suspension, the pH of the liquid is 4.0-5.0. The relative labeling amount of dafloxacin mesylate is 99.39%, and the appearance properties have no obvious change during the high temperature and light test, and the content change is within 0.2%. The sedimentation volume ratio is more than 0.9, it can be re-dispersed after standing for several days or months, and it can be re-dispersed after vigorous shaking.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com