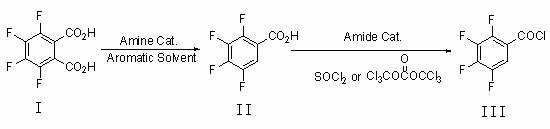
Preparation method of 2,3,4,5-tetrafluorobenzoyl chloride
A technology of tetrafluorobenzoyl chloride and tetrafluorobenzoic acid is applied in the field of preparation of 2,3,4,5-tetrafluorobenzoyl chloride, and can solve the problems of complicated operation process, long production cycle, and many product losses, etc., To achieve the effect of simplifying the operation process, reducing production time and avoiding product loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 47.6 grams of 3,4,5,6-tetrafluorophthalic acid (I), 285 grams of trimethylbenzene, and 1.5 grams of tri-n-propylamine into a 500 mL three-necked flask equipped with mechanical stirring, a thermometer, and a condenser, and stir to raise the temperature to 150 o C, reacted for 15 hours, and the reaction system was cooled to 20-30 o C, filtered to obtain 39.5 g of crude 2,3,4,5-tetrafluorobenzoic acid (II), and the filtrate was used for the next batch of decarboxylation reaction.
Embodiment 2
[0030] Add 47.6 grams of 3,4,5,6-tetrafluorophthalic acid (I) and 1.5 grams of tri-n-propylamine into a 500 mL three-necked flask equipped with mechanical stirring, a thermometer, and a condenser, and add the recovered Trimethylbenzene, and add trimethylbenzene to 285 grams, stir and heat up to 150 o C, reacted for 15 hours, and the reaction system was cooled to 20-30 o C, filtered to obtain 42.2 g of crude 2,3,4,5-tetrafluorobenzoic acid (II), and the filtrate was used for the next batch of decarboxylation reaction.
Embodiment 3
[0032] Add 35.7 grams of 3,4,5,6-tetrafluorophthalic acid (I), 320 grams of xylene, and 1.5 grams of tri-n-butylamine into a 500 mL three-neck flask equipped with mechanical stirring, a thermometer, and a condenser, and stir Warm up to 135 o C, reacted for 22 hours, and the reaction system was cooled to 20-30 o C, filtered to obtain 30.3 g of 2,3,4,5-tetrafluorobenzoic acid (II), and the filtrate was used for the next batch of decarboxylation reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com