Preparation method and application of tamoxifen twin drug
A technology of tamoxifen and twin drugs, which is applied in the field of new tamoxifen twin drugs and its preparation, can solve the problems of threatening women's lives and unsatisfactory overall treatment effects, and achieve improved specificity, reduced dosage, The effect of reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
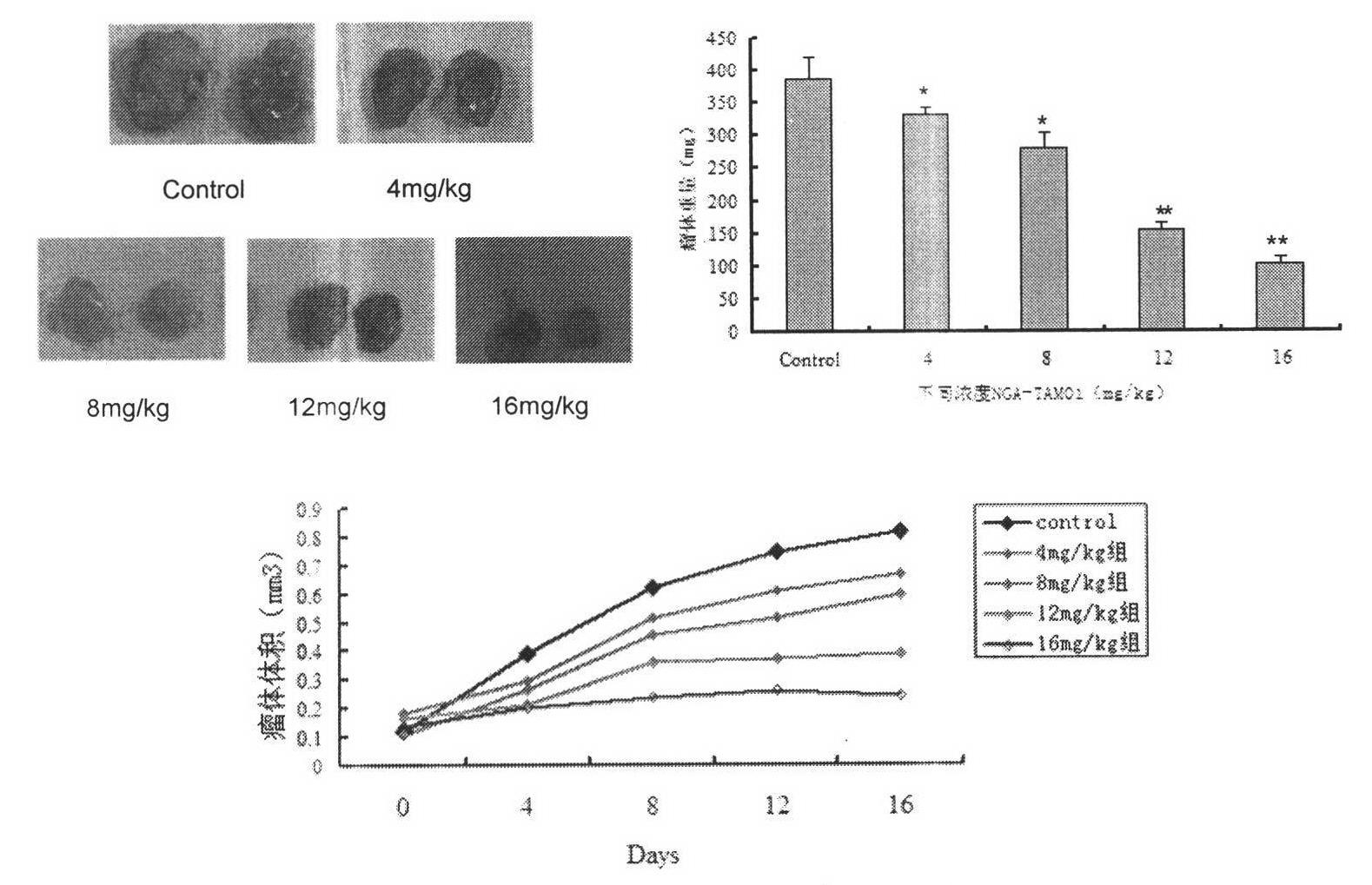
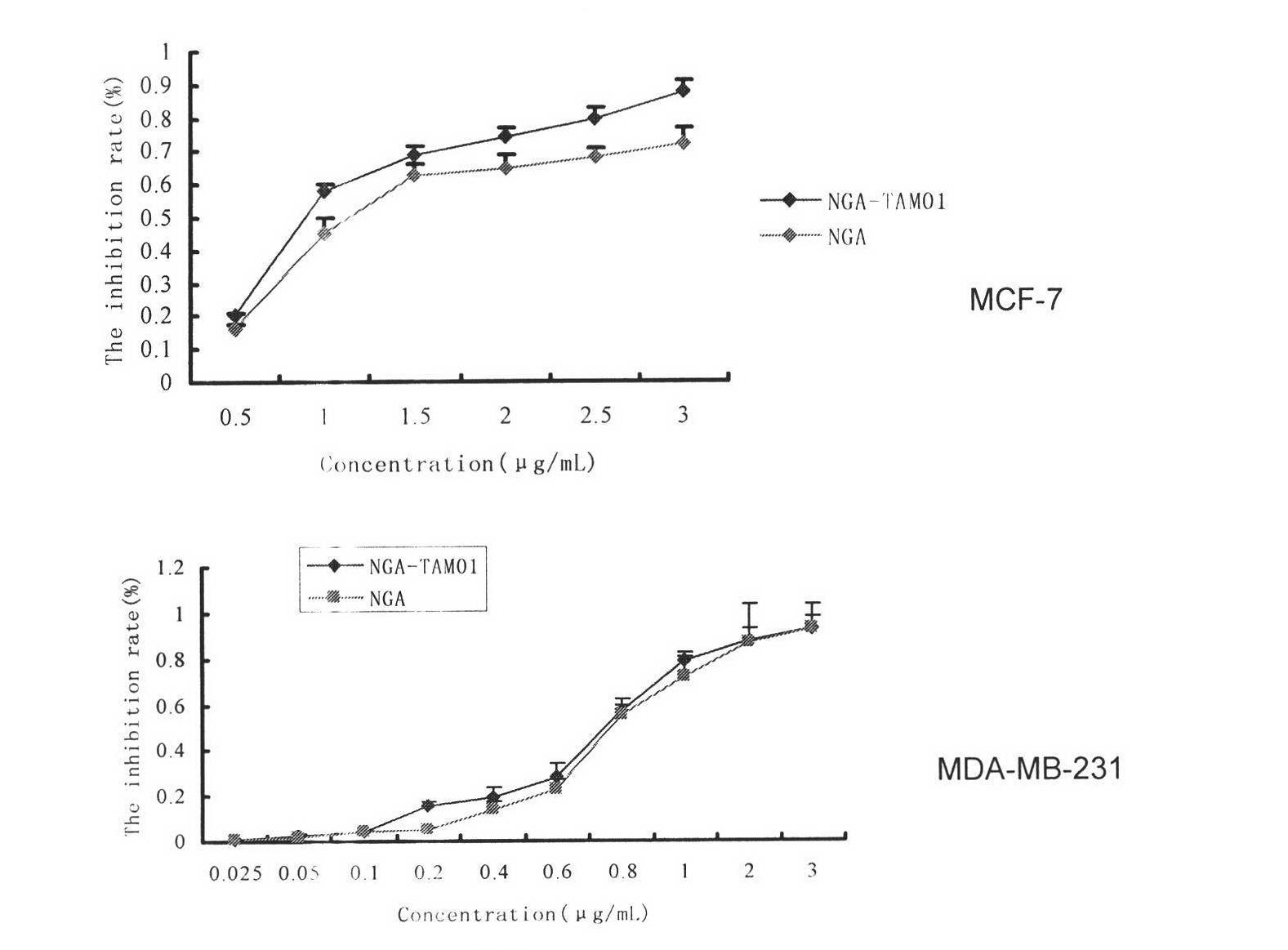
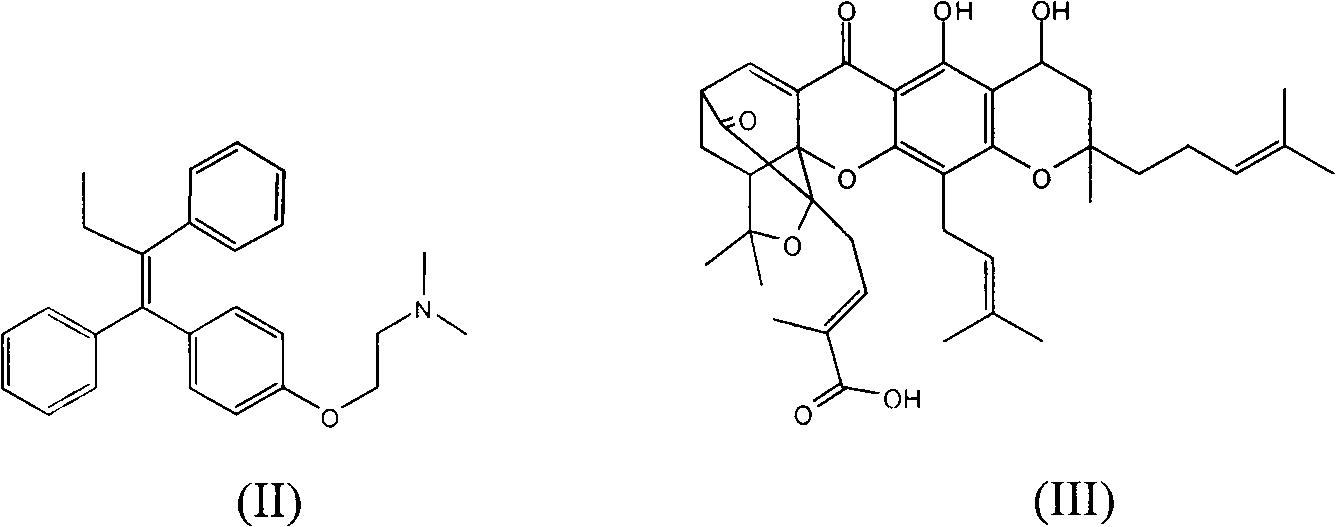
Image
Examples
Embodiment 1
[0019] Synthesis of 4-Hydroxy-4'-(trimethylacetoxy)benzophenone and 4,4'-bis(trimethylacetoxy)benzophenone
[0020] Dissolve 4,4'-dihydroxybenzophenone (7.5g, 35mmol) in dry THF (100mL), add NaH (2.5g, 38mmol), stir at room temperature for 1h, cool to 0°C, add PvCl (5mL), Remove the ice-water bath and stir for 3h. The reaction mixture was poured into distilled water (100 mL) and extracted with ethyl acetate (3 x 100 mL). Combined organic phases, anhydrous Na 2 SO 4 Dry overnight and concentrate. The two compounds were separated by column chromatography (hexane-dichloromethane 9:1), and both compounds were white powders. Wherein 4-hydroxy-4'-(trimethylacetoxy) benzophenone, 1H NMR (δ, CDCl3): 1.32 (s, 9H), 6.830 (d, 2H), 7.19 (d, 2H), 7.72 (d, 2H), 7.79 (d, 2H); 4,4'-two (trimethylacetoxy) benzophenone, 1H NMR (δ, CDCl3): 1.20 (s, 18H), 7.22 ( d, 4H), 7.82 (d, 4H).
Embodiment 2
[0022] Synthesis of (E)-4-(1-(4-hydroxyphenyl)-2-phenyl-1-enyl)phenyl pivalate
[0023] Disperse Zn powder (1.0g, 15mmol) in 20mL dry THF, add TiCl4 (0.8mL, 7mmol) dropwise under argon protection, reflux for 3h, add 20mL containing 4-hydroxy-4'-(trimethylacetoxy ) a solution of benzophenone (1.0 g, 3.5 mmol) and propiophenone (1.0 mL, 11 mmol) in dry THF. Reflux for 5h, cool to room temperature, add 10% K 2 CO 3 (40 mL), extracted with ethyl acetate (3 x 100 mL). Combine the organic phases to 10% K 2 CO 3 (2×50 mL) and saturated NaCl solution (50 mL), concentrated, and recrystallized from methanol to obtain a white powder. E / Z ratio greater than 100:1. 1H NMR (δ, CD3OD): 0.95(t, 3H), 1.26(s, 9H), 2.26(q, 2H), 6.40(d, 2H), 6.66(d, 2H ), 7.22-7.45(m, 9H)
Embodiment 3
[0025] Synthesis of (E)-4-[1-(4-(2-(N,N-dimethylamino)ethoxy)phenyl)-2-phenylpropyl-1-ene]phenylpivalate Get the product (0.60g, 1.5mmol) in Example 2, fresh distillation 2-dimethylaminochloroethane (0.32g, 3.0mmol) and K 2 CO 3 (0.27g, 1.9mmol) was poured into a mixed solution of acetone:water (19:1, 20mL), and refluxed for 6h in the dark. Cool to room temperature, dry, evaporate the solvent, separate by column chromatography (dichloromethane-methanol 19:1), and recrystallize from isopropanol to obtain a white powder. 1H NMR (δ, CDCl3): 0.95(t, 3H), 1.28(s, 9H), 2.29(s, 6H), 2.46(q, 2H), 2.68(t, 2H), 3.93(t, 2H), 6.56(d, 2H), 6.80(d, 2H), 7.14-7.32(m, 9H);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com