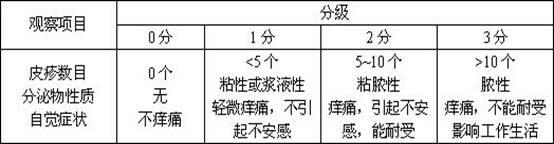
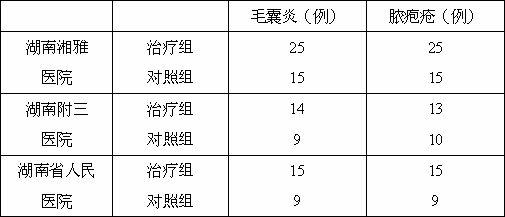
Lomefloxacin hydrochloride emulsifiable paste and preparation method thereof
A technology of lomefloxacin hydrochloride and lomefloxacin cream, which is applied in the direction of ointment delivery, pharmaceutical formula, emulsion delivery, etc., can solve the problems of insufficient function and low osmotic concentration of the affected part, so as to avoid adverse reactions and drug The effect of high concentration and stable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Take 10.8g of stearic acid, 4.8g of glyceryl monostearate, 2.4g of liquid paraffin, 1.6g of white vaseline, 0.8g of Span-80 and 0.1g of benzoic acid in the same container and heat and stir to melt. The temperature is 75-80°C; add 1.1g of lomefloxacin hydrochloride and 0.04g of disodium edetate into 72g of distilled water, heat to dissolve, add 4g of glycerin, 2g of triethanolamine, and 2g of Tween-80 and stir well, and control the temperature at 75-80°C; when the temperature of the two phases is the same, add the water phase to the oil phase, stir while adding, and finally add an appropriate amount of essence, stir well to 50±5°C, after the content is qualified, divide the package, and the product is ready.
Embodiment 2
[0022] Take 21.6g of stearic acid, 9.6g of glyceryl monostearate, 4.8g of liquid paraffin, 3.2g of white petrolatum, 1.6g of Span-80 and 0.2g of benzoic acid in the oil phase and put them together in the same container for heating, stirring and melting. The temperature is 75-80°C; add 2.2g of lomefloxacin hydrochloride and 0.08g of disodium edetate into 144g of distilled water, heat and dissolve, add 8g of glycerin, 4g of triethanolamine, and 4g of Tween-80 and stir well, and control the temperature at 75-80°C; when the temperature of the two phases is the same, add the water phase to the oil phase, stir while adding, and finally add an appropriate amount of essence, stir until the temperature is 50±5°C, after the content of the test is qualified, then pack it, that is have to.
Embodiment 3
[0024] Take 54g of stearic acid, 24g of glyceryl monostearate, 12g of liquid paraffin, 8g of white petrolatum, 4g of Span-80 and 0.5g of benzoic acid in the oil phase and put them together in the same container for heating, stirring and melting, and control the temperature at 75-80 ℃; Add 5.53g of lomefloxacin hydrochloride and 0.2g of disodium edetate into 360g of distilled water, heat to dissolve, add 20g of glycerin, 10g of triethanolamine, and 10g of Tween-80 and stir well, and control the temperature at 75-80°C; When the temperature of the two phases is the same, add the water phase to the oil phase, stir while adding, and finally add an appropriate amount of essence, stir well until the temperature is 50±5°C, after the content is qualified, divide the package to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com