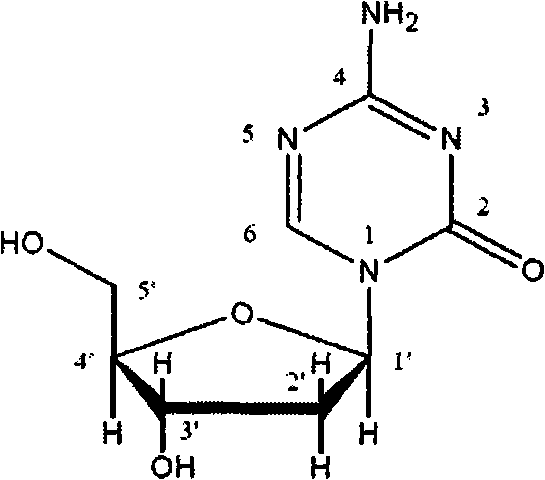
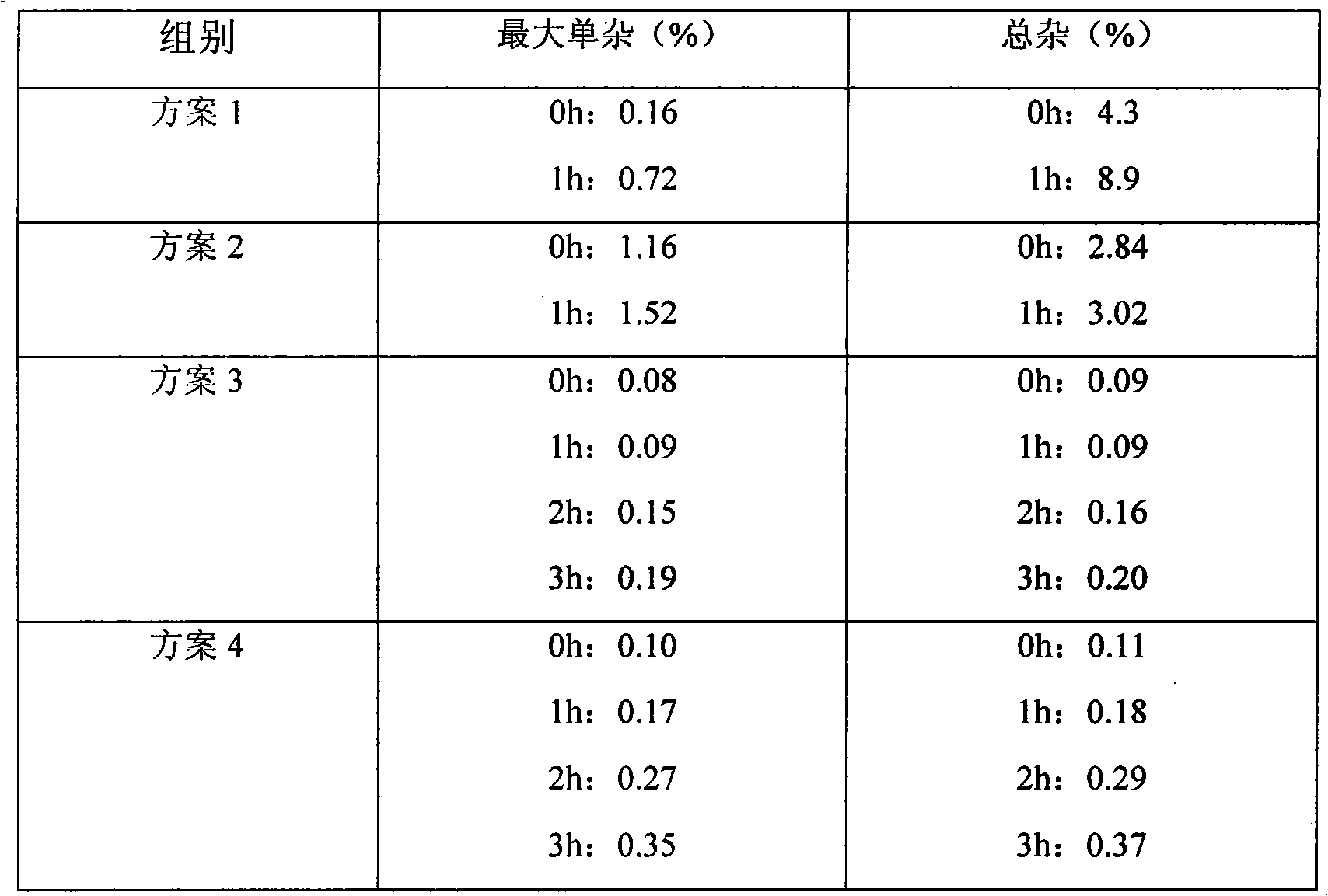
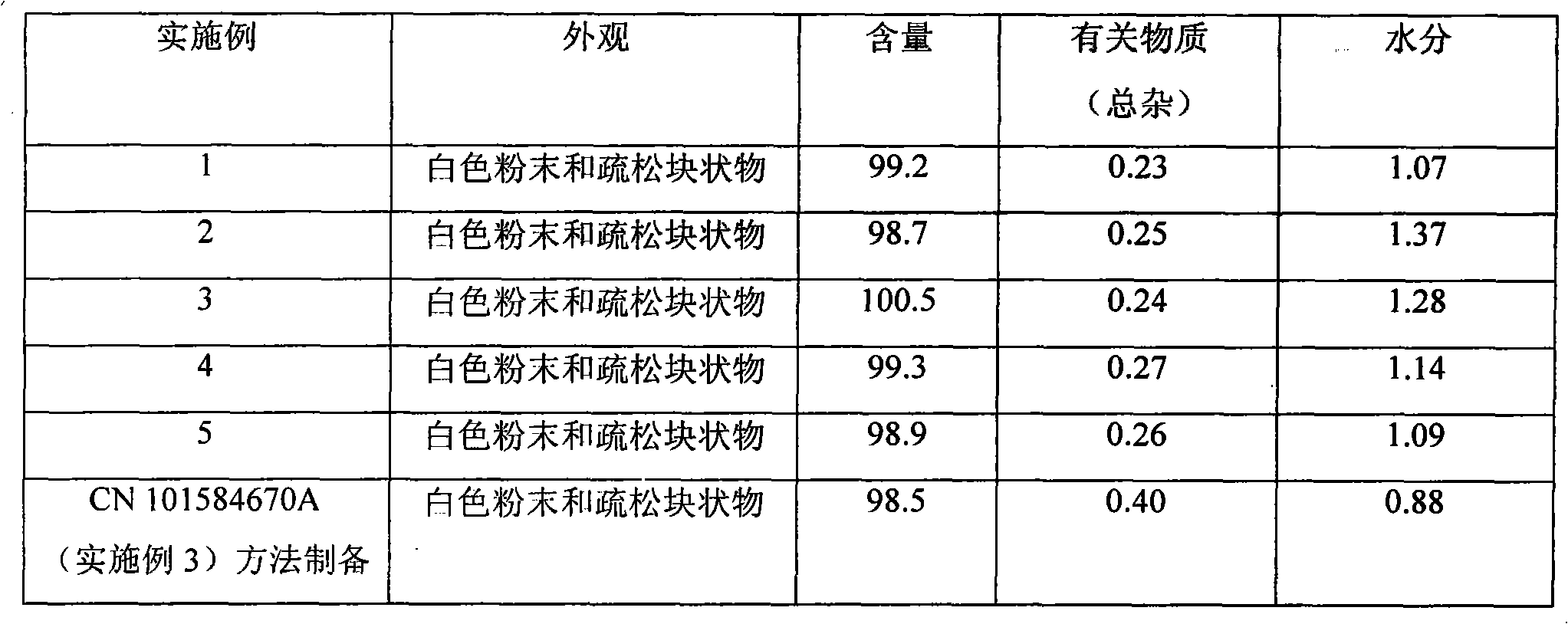
Decitabine liquid composition and preparation method of its lyophilized preparation
The technology of a liquid composition and decitabine, which is applied in the field of medicine, can solve problems such as high requirements for instruments and operating environment, difficult operation, and large amount of solvent, and achieve simplified production equipment process and cost, improved solubility, and related substances The effect of low content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] prescription
[0028] Decitabine 50g
[0029] Potassium dihydrogen phosphate 68g
[0031] Ethanol 250ml
[0032] Water for injection 9.75L
[0033]
[0034] A total of 1000 bottles were made
[0035] Preparation Process:
[0036] 1. Dissolve potassium dihydrogen phosphate and sodium hydroxide in water for injection.
[0037] 2. Uniformly disperse decitabine in the prescribed amount of organic solvent ethanol.
[0038] 3. Mix and stir the two phases at room temperature to dissolve, add activated carbon, filter, fill, and freeze-dry.
[0039] 4. Start the freeze dryer, after pre-freezing at -45°C for 5 hours, turn on the vacuum pump, raise the temperature to -20°C, and keep it for 7 hours; the second step is to raise the temperature to 0°C, keep it for 9 hours; then raise the temperature to 40°C and dry for 6 hours.
[0040] 5. Vacuum stopper, aluminum cover.
[0041] 6. After passing the ...
Embodiment 2
[0043] prescription
[0044] Decitabine 50g
[0045] Potassium dihydrogen phosphate 68g
[0046] Sodium hydroxide 11.6g
[0047] Ethanol 200ml
[0048] Mannitol 200g
[0050] Water for injection 9.8L
[0051]
[0052] A total of 1000 bottles were made
[0053] Preparation Process:
[0054] 1. Dissolve potassium dihydrogen phosphate, sodium hydroxide, mannitol, and sodium bisulfite in water for injection.
[0055] 2. Uniformly disperse decitabine in the prescribed amount of organic solvent ethanol.
[0056] 3. Mix and stir the two phases at room temperature to dissolve, add activated carbon, filter, fill, and freeze-dry.
[0057] 4. Start the freeze dryer, after pre-freezing at -45°C for 5 hours, turn on the vacuum pump, raise the temperature to -20°C, and keep it for 7 hours; the second step is to raise the temperature to 0°C, keep it for 9 hours; then raise the temperature to 40°C and dry...
Embodiment 3
[0061] prescription
[0062] Decitabine 50g
[0063] Potassium dihydrogen phosphate 68g
[0064] Sodium hydroxide 11.6g
[0065] Ethanol 250ml
[0066] Glycine 125g
[0067] EDTA-2Na 5g
[0068] Water for injection 9.75L
[0069]
[0070] A total of 1000 bottles were made
[0071] Preparation Process:
[0072] 1. Dissolve potassium dihydrogen phosphate, sodium hydroxide, glycine, EDTA-2Na in water for injection.
[0073] 2. Uniformly disperse decitabine in the prescribed amount of organic solvent ethanol.
[0074] 3. Mix and stir the two phases at room temperature to dissolve, add activated carbon, filter, fill, and freeze-dry.
[0075] 4. Start the freeze dryer, after pre-freezing at -45°C for 5 hours, turn on the vacuum pump, raise the temperature to -20°C, and keep it for 7 hours; the second step is to raise the temperature to 0°C, keep it for 9 hours; then raise the temperature to 40°C and dry for 6 hours.
[007...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com