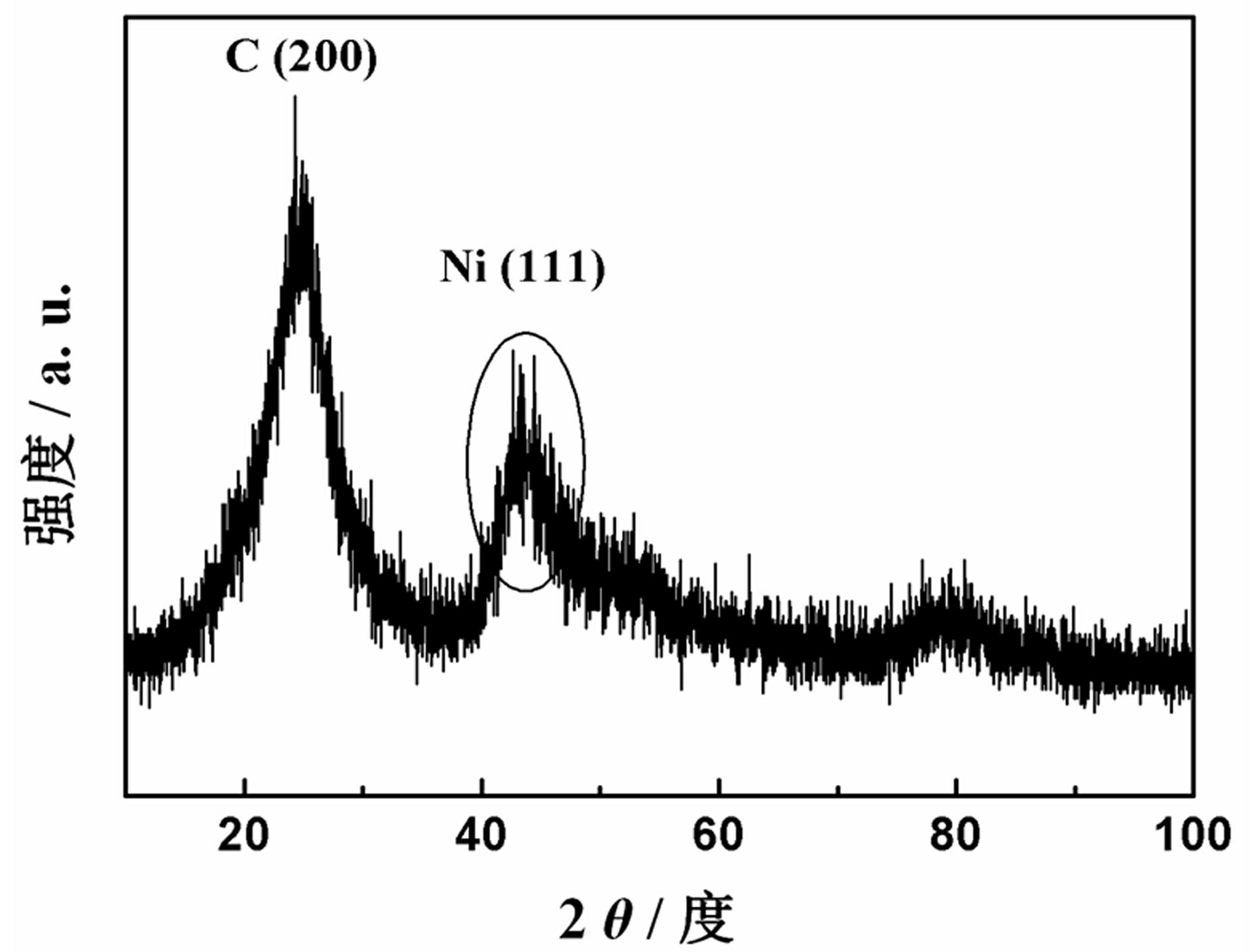
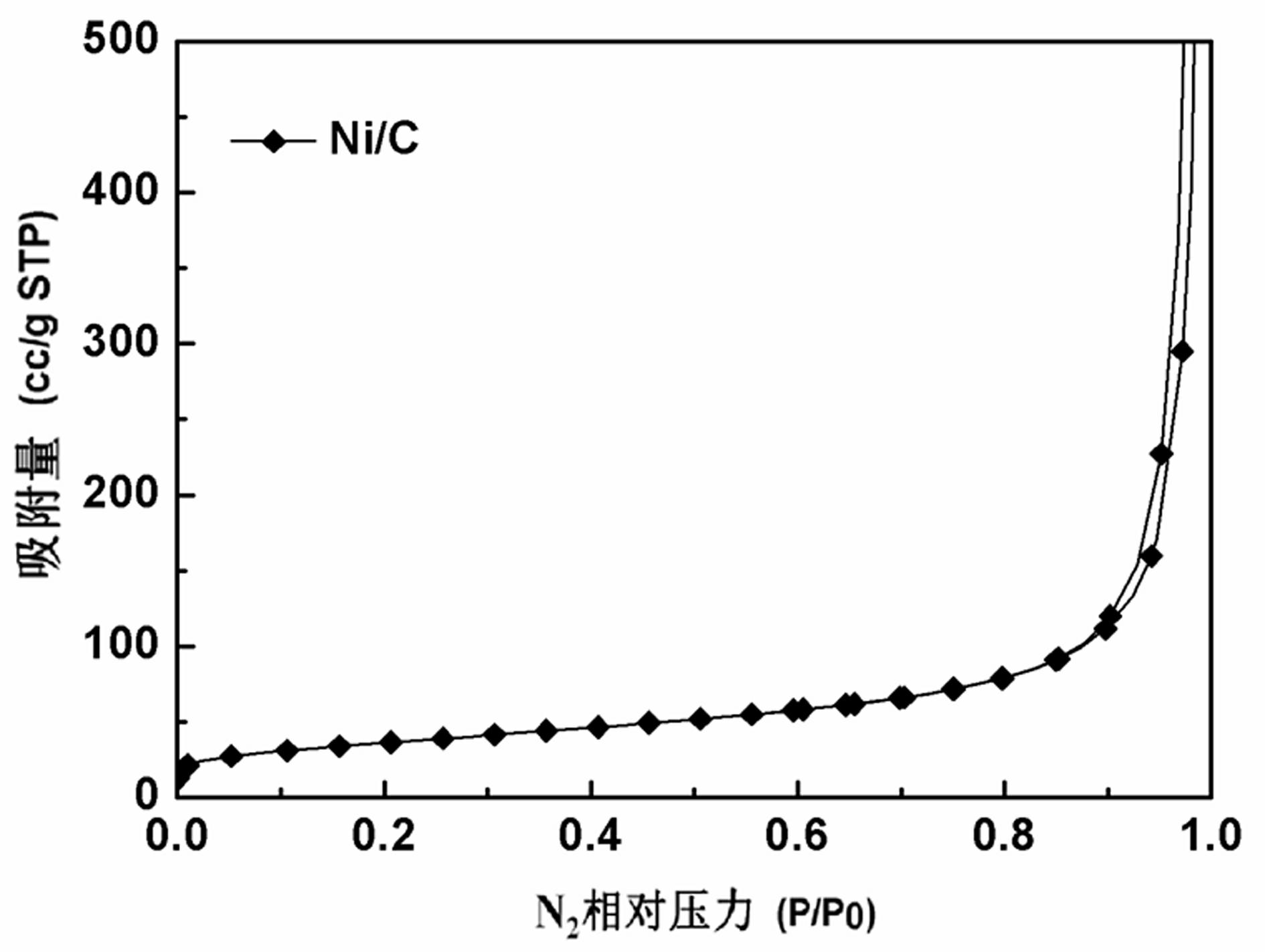
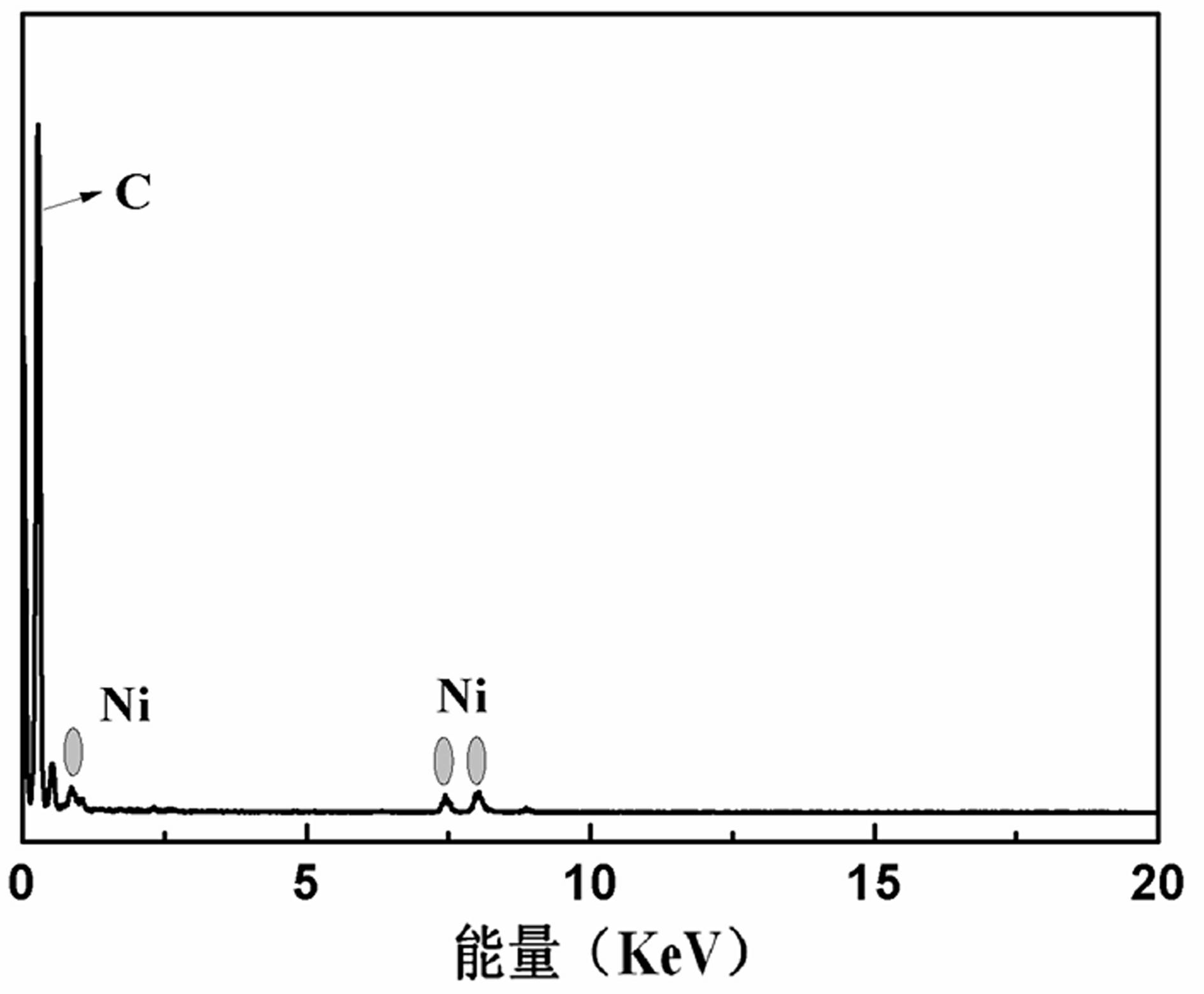
Preparation and application of carbon-supported amorphous metallic nickel
An amorphous, metallic nickel technology, applied in the field of metal materials, can solve the problems of restricting the commercial development of fuel cells, catalyst poisoning failure, expensive Pt, etc., to achieve enhanced resistance to CO poisoning, good catalytic activity, and long service life. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Preparation of carbon-supported amorphous metal Ni / C
[0035] Add 25 ml of ethanol and 25 ml of water three times into a 100 ml round bottom flask, and pass N 2 After two hours, add 0.8 g ethylenediaminetetraacetic acid (EDTA), stir and ultrasonic to dissolve, then add 0.3 g NiCl 2 · 6H 2 O, after stirring, add 2 g of KOH to adjust the reaction solution to pH=14, then add 15ml of hydrazine hydrate (80%) and 0.3g of carbon carrier (Vulcan XC-72R) to the round bottom flask, stir and sonicate, the process is all Pass N 2 Under the conditions. Afterwards, the reaction solution was put into the reaction kettle and reacted at 120° C. for 4 hours to obtain a black amorphous metal Ni / C slurry solution. Suction filtration, rapid cooling in cold water, and vacuum drying at 50~60℃ to obtain carbon-supported amorphous metal Ni.
[0036] In carbon-supported amorphous metal nickel Ni / C, the mass of metal Ni is 20% of the mass of carbon-supported amorphous metal nickel Ni / C.
[0037] (...
Embodiment 2
[0041] (1) Preparation of amorphous metal Ni / C
[0042] Add 25 ml of ethanol and 25 ml of water three times into a 100 ml round bottom flask, and pass N 2 After two hours, add 0.8 g ethylenediaminetetraacetic acid (EDTA), stir and ultrasonic to dissolve, then add 0.3 g NiCl 2 · 6H 2 O, after stirring, add 3 g of KOH to adjust the reaction solution to pH=13, then add 15 ml of hydrazine hydrate (80%) and 0.3 g of carbon carrier (Vulcan XC-72R) to the round bottom flask, stir and sonicate, the process is all At pass N 2 Under the conditions. Afterwards, the reaction liquid was put into the reaction kettle and reacted at 90° C. for 5 hours to obtain a black amorphous metal Ni / C slurry solution. Suction filtration, rapid cooling in cold water, and vacuum drying at 50~60℃ to obtain carbon-supported amorphous metal Ni.
[0043] (2) Preparation of NiPd / C catalyst
[0044] Add 30 mg PdCl to the flask 2 Dissolve with a small amount of concentrated hydrochloric acid and ultrasonically, then a...
Embodiment 3
[0047] (1) Preparation of amorphous metal Ni / C
[0048] Add 25 ml of ethanol and 25 ml of water three times into a 100 ml round bottom flask, and pass N 2 After two hours, add 0.8 g ethylenediaminetetraacetic acid (EDTA), stir and ultrasonic to dissolve, then add 0.3 g NiCl 2 · 6H 2 O, after stirring, add 2 g of KOH to adjust the reaction solution to pH=12, then add 15 ml of hydrazine hydrate (80%) and 0.45 g of carbon carrier (Vulcan XC-72R) into the round bottom flask, stir and sonicate, the process is all At pass N 2 Under the conditions. Then, the reaction solution was put into the reaction kettle and reacted at 160° C. for 4 hours to obtain a black amorphous metal Ni / C slurry solution. Suction filtration, rapid cooling in cold water, and vacuum drying at 50~60℃ to obtain carbon-supported amorphous metal Ni.
[0049] (2) Preparation of NiPtRu / C catalyst
[0050] Add 30 ml EG, 5.6 ml 20 mg / ml H in the flask 2 PtCl 6 With 2.9mL 20 mg / ml RuCl 3 · XH 2 O, stir to dissolve, adjust p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com