Application of tamoxifen, rapamycin and ginsenoside Rg3 composition in preparing medicaments for treating liver cancer
A technology of tamoxifen and ginsenosides, which is applied in the directions of drug combination, antitumor drug, pharmaceutical formula, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1. Reagents: RPMI-1640 medium was produced by GIBCOL BRL, USA; mouse anti-survivin, p70s6k Thr389 monoclonal antibody was purchased from Cell Signal; secondary antibody HRP-labeled anti-mouse lgG, chemiluminescence (ECL) substrate was purchased from Santa Cruz Company; Tamoxifen, rapamycin, and ginsenoside Rg3 were purchased from Sigma, Renilla luciferase plasmid pRL-CMV was purchased from Promega. The plasmid pLUC encoding the firefly luciferase gene and survivin promoter sequence was kindly given by Dr. Xun from the University of North Carolina.
[0020] 2. Cell culture: Human liver cancer HepG2 cell line is routinely cultured in RPMI-1640 culture medium, with 10% calf serum, human penicillin (concentration 100 U / ml) and streptomycin (concentration 100 μg / ml), 37 ℃、5%CO 2 Cultivate in an incubator. After 3 to 4 generations of adherent growth in the incubator, cells in the logarithmic growth phase were used for experiments.
[0021] 3. Grouping: The logarithmic growth ph...
Embodiment 2
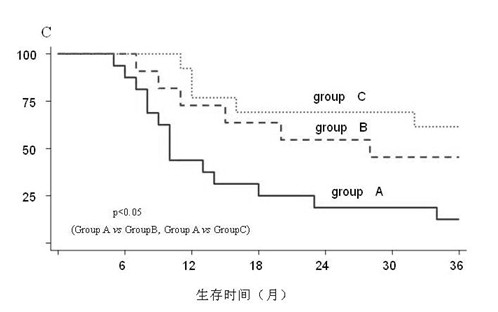
[0033] Tamoxifen has achieved significant effects in inhibiting tumor growth in breast and liver cancer experiments. Tamoxifen combined with rapamycin and ginsenoside Rg3 can enhance the effect of its cytotoxic drugs. Therefore, the combination therapy of tamoxifen, rapamycin and ginsenoside Rg3 will provide an efficient, low-toxic and inexpensive method for the treatment of liver cancer.
[0034] We have carried out clinical testing for the expression of survivin, p-Akt, and ER in tissue specimens after liver cancer surgery. Part of the patients with positive expression of survivin and p-Akt have been partially treated with moxifen combined with rapamycin and ginsenoside during outpatient follow-up. Rg3, tamoxifen monotherapy and controlled observation follow-up, at least 2 courses of treatment. The results showed that: compared with the single-drug treatment group and the observation group (not administered in the control group), the three-drug combination treatment group (moxi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com