Preparation method of phencyclidine artificial antigen
An artificial antigen, phencyclidine technology, applied in the direction of peptide preparation methods, chemical instruments and methods, specific peptides, etc., can solve the problems of expensive instruments, time-consuming detection, and unreachable, and achieve advanced synthesis technology and strong specificity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
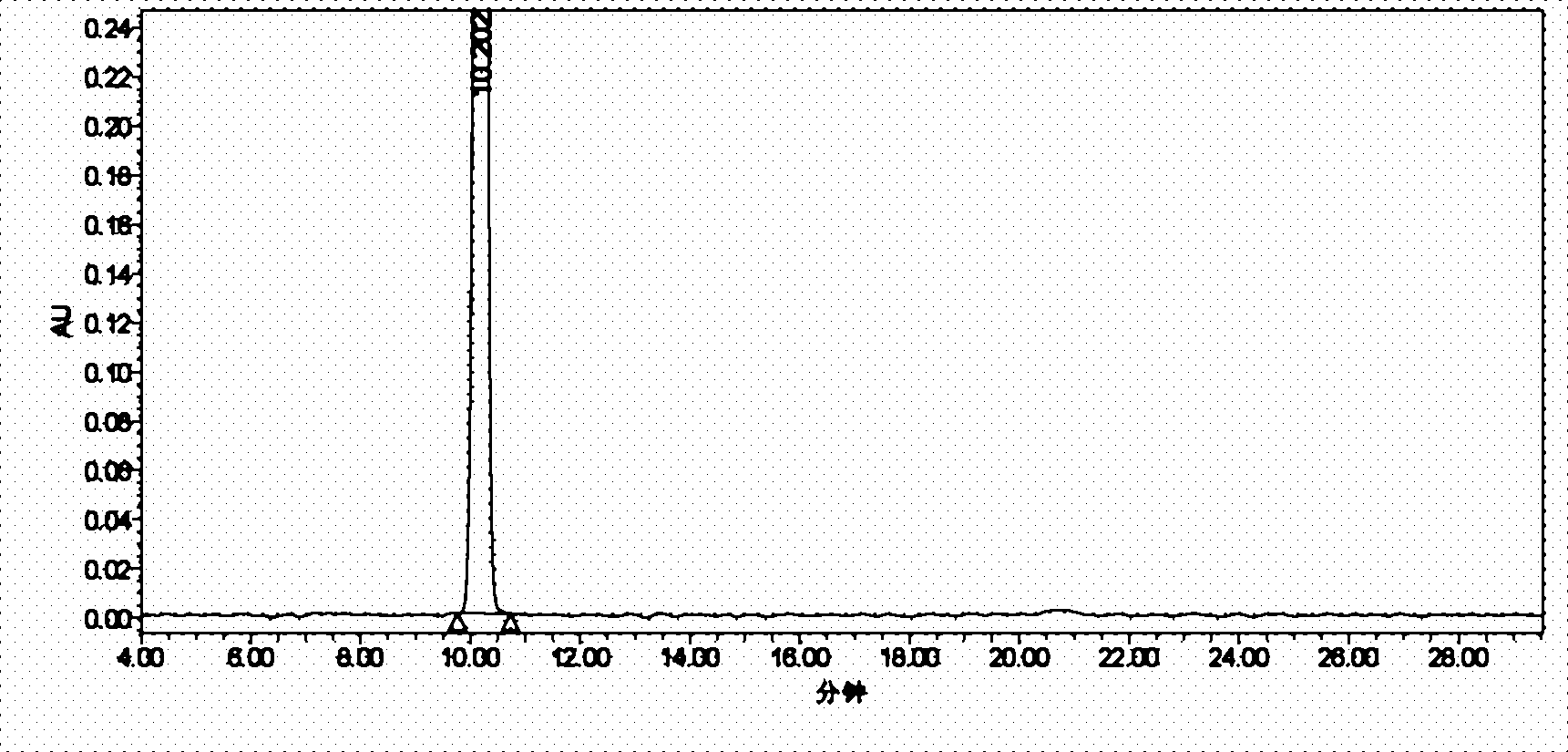
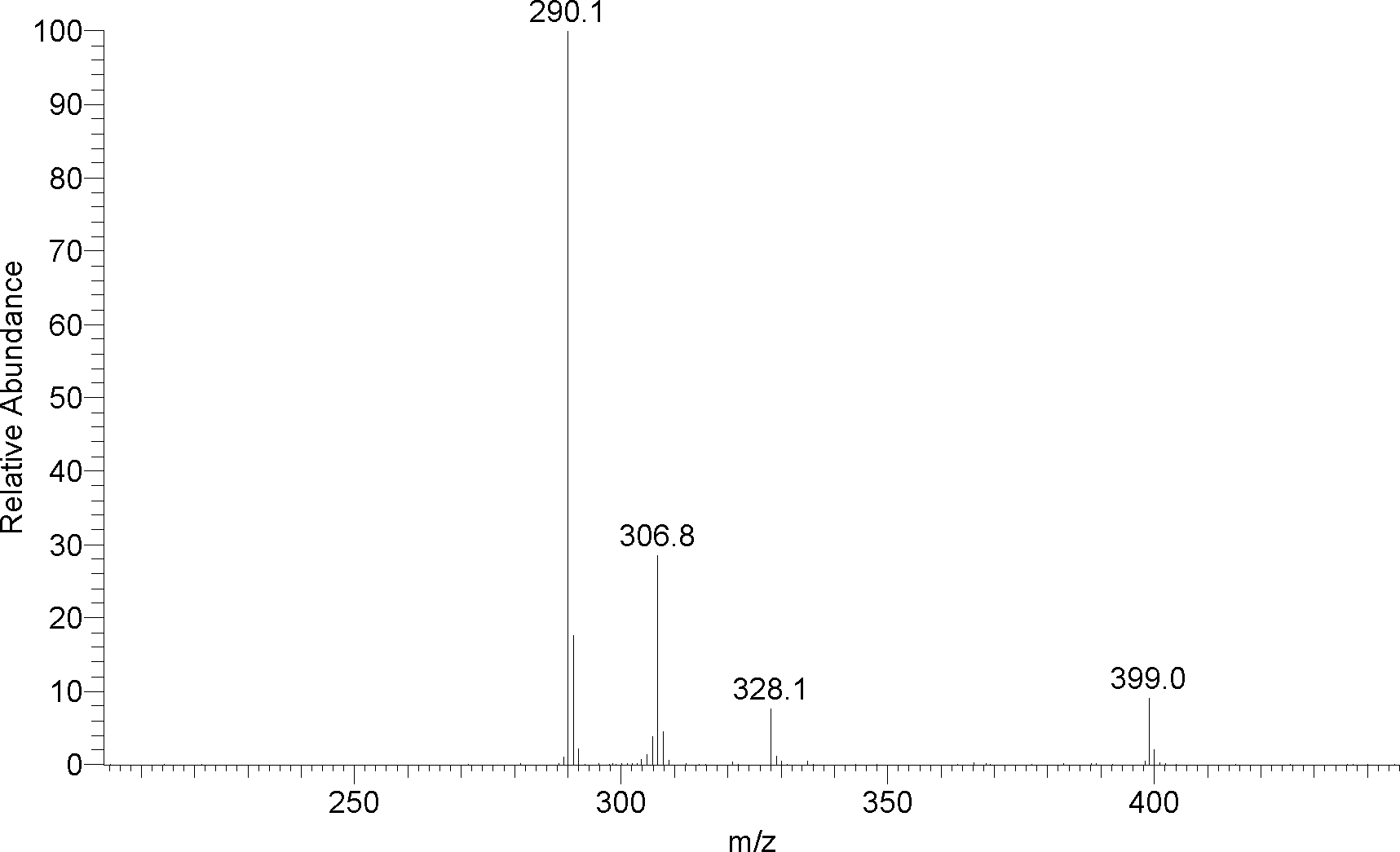
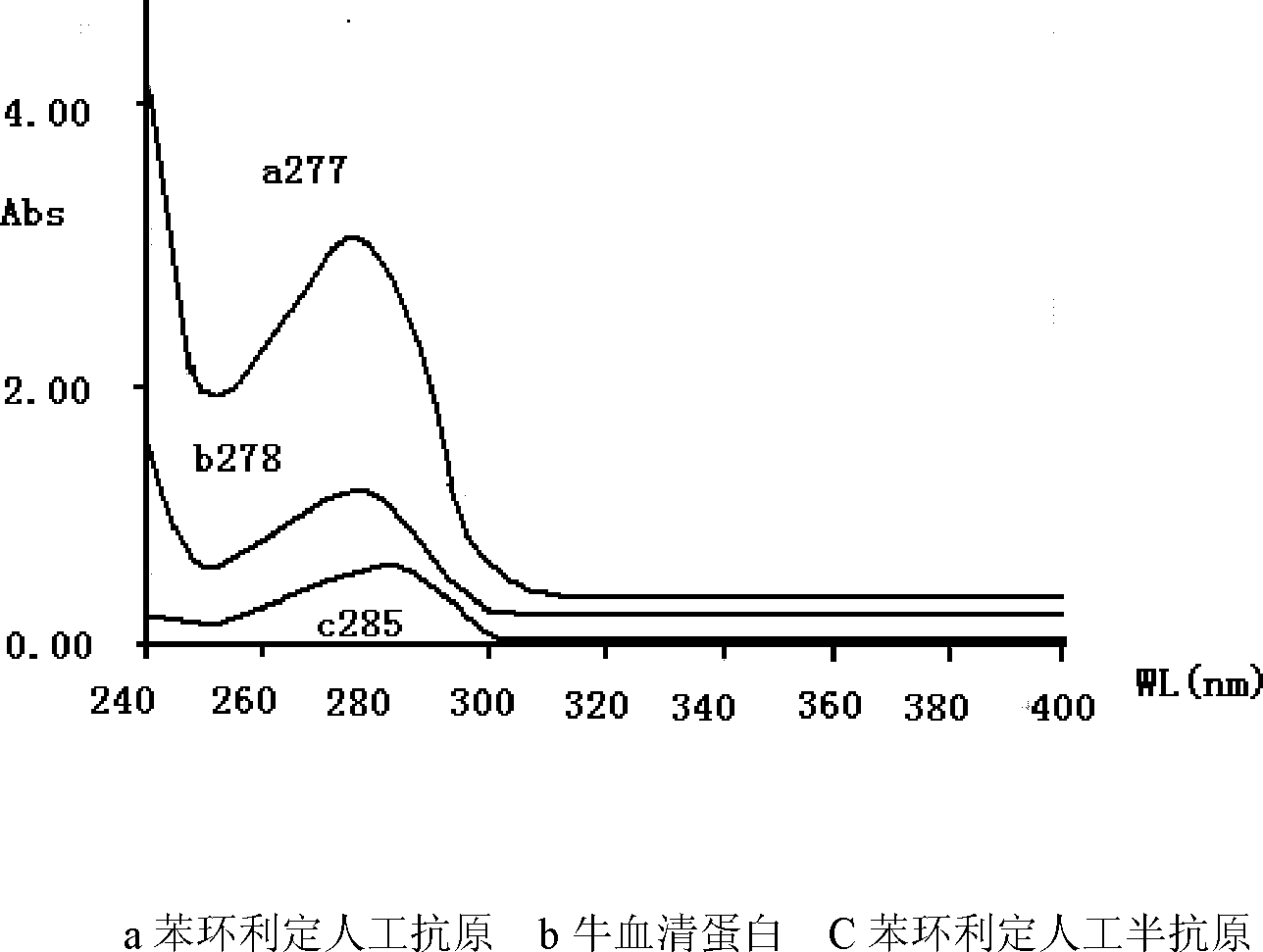
Image
Examples
Embodiment 1
[0033] (1) Preparation of artificial hapten:
[0034] (a) Weigh 2.024g (11.5mmol) of 1-phenylcyclohexanol into a 100ml three-neck round bottom flask, add 40ml of chloroform, and stir at room temperature until the solid is completely dissolved; another 1.740g (23mmol) of sodium azide is weighed Add it to the above reaction solution, cool it to about 0°C with an ice bath, start to slowly add 5ml of trifluoroacetic acid, remove the ice bath after the addition, and stir at room temperature for 5 hours. After the reaction, use concentrated ammonia water to adjust the pH to about 9. After standing and stratifying, the organic phase is washed with double distilled water and saturated aqueous sodium chloride solution. The organic layer is distilled under reduced pressure to obtain 2.023 g (10 mmol) of azide-containing compounds. . Thin-layer chromatography: the chromatographic solution is n-hexane:ethyl acetate=4:1, the product R f =0.7~0.8.
[0035](b) Weigh 0.570g of lithium al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com