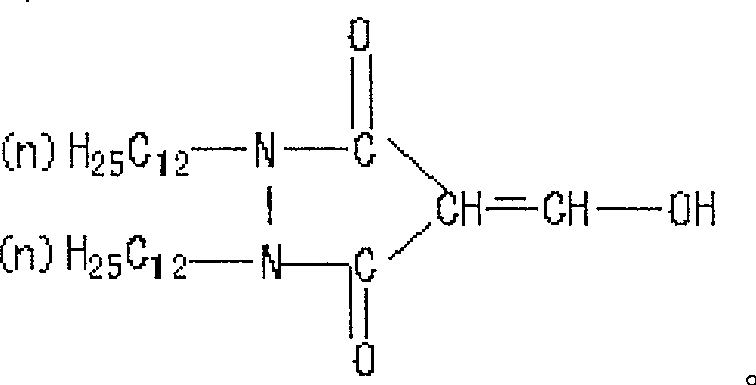
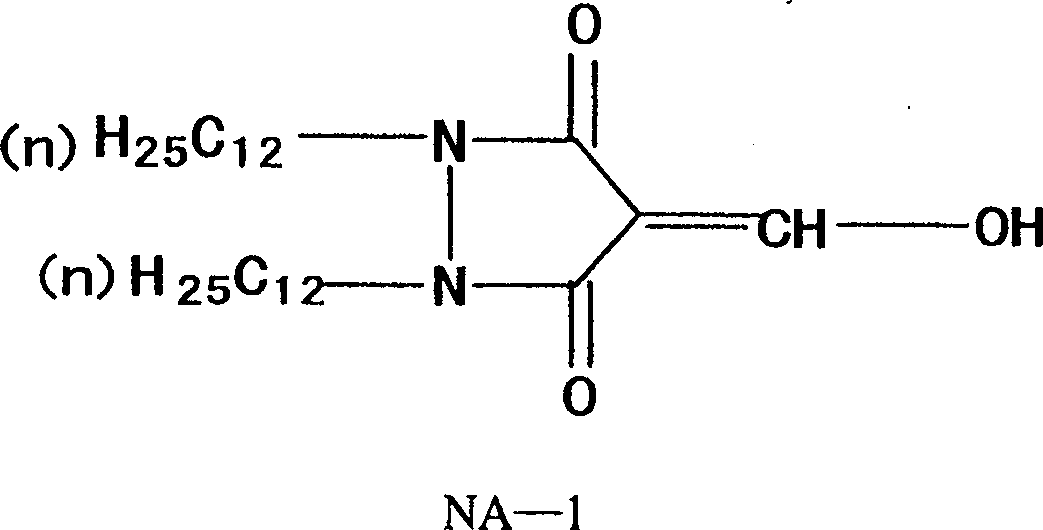
Method for preparing compound of 1, 2 (dodecyl) 4 hydroxide methylene - 3, 5 pyrazole
A technology of pyrazolidinedione and hydroxymethylene, which is applied in 1 field, can solve problems such as the synthesis of undisclosed compounds, and achieve the effects of simple post-processing, high product purity, and advanced synthesis technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a 1000mL three-necked flask, add 105ml of dodecanal, 45g of calcium oxide, 15ml of hydrazine hydrate (85% content) and 120mL of absolute ethanol, reflux reaction for 2 hours, cooling, and precipitate 64g of intermediate A, yield 73 %;
[0022] 64 grams of intermediate A and 17.5 grams of lithium aluminum hydride were refluxed in 600 milliliters of anhydrous ether for 2 hours, and 10% aqueous sodium hydroxide solution was added until no obvious hydrogen gas was released. After cooling, 60 grams of white solid was precipitated, which was intermediate B , yield 93%;
[0023] 60 grams of intermediate B and 17.5 grams of 30% sodium hydride, 39 milliliters of diethyl malonate were refluxed in 140 milliliters of chlorobenzene for 5 hours, and the ethanol generated in the reaction was evaporated under reduced pressure, and 265 milliliters of dichloromethane and 265 milliliters of water was extracted, and 2N hydrochloric acid was added to the extract to adjust the pH value t...
Embodiment 2
[0030] In a 500mL three-neck flask, add 53ml of dodecanal, 23g of calcium oxide, 8ml of hydrazine hydrate (85% content) and 80mL of methanol, reflux for 1.5 hours, cool, and precipitate 30g of intermediate A, with a yield of 68%;
[0031] 30 grams of intermediate A and 8.4 grams of lithium aluminum hydride were refluxed in 250 ml of anhydrous ether for 1 hour, and 15% potassium hydroxide aqueous solution was added until no obvious hydrogen gas was released. After cooling, 28 grams of white solid were precipitated, which was intermediate B , yield 92%;
[0032] 28 grams of intermediate B and 8.4 grams of 30% sodium hydride, 18 milliliters of diethyl malonate were refluxed in 70 milliliters of toluene for 4.5 hours, and the ethanol generated in the reaction was evaporated under reduced pressure, and 120 milliliters of chloroform and 120 milliliter of water for extraction, adding 1N sulfuric acid to the extract to adjust the pH value to 4.5, and 19.7 grams of intermediate C were ...
Embodiment 3
[0039] In a 500mL three-necked flask, add 60 milliliters of dodecanal, 26 grams of calcium oxide, 10 milliliters of hydrazine hydrate (85% content) and 70 milliliters of dehydrated ethanol, reflux for 1 hour, cool, and separate out 35 grams of intermediate A with a yield of 70 %;
[0040] 35 grams of intermediate A and 10 grams of lithium aluminum hydride were refluxed in 350 milliliters of anhydrous ether for 2 hours, and 10% aqueous sodium hydroxide solution was added until no obvious hydrogen gas was released. After cooling, 32 grams of white solid were precipitated, which was intermediate B , yield 91%;
[0041] 32 grams of intermediate B and 10 grams of 30% sodium hydride, 21 milliliters of diethyl malonate were refluxed in 80 milliliters of xylene for 4 hours, and the ethanol generated in the reaction was evaporated under reduced pressure, and 150 milliliters of normal hexane and 100 Milliliter of water was extracted, and 50% glacial acetic acid was added to the extract...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com