Magenta dye and preparation method and application thereof
A dye, magenta technology, applied in the field of magenta dye and its preparation, can solve the problems that magenta dye cannot meet the hue, vividness, light fastness, water resistance, ozone resistance, solubility and solution stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
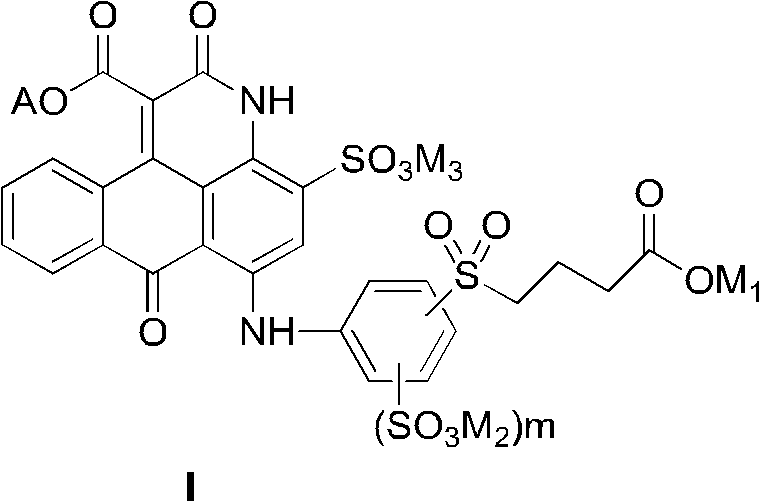
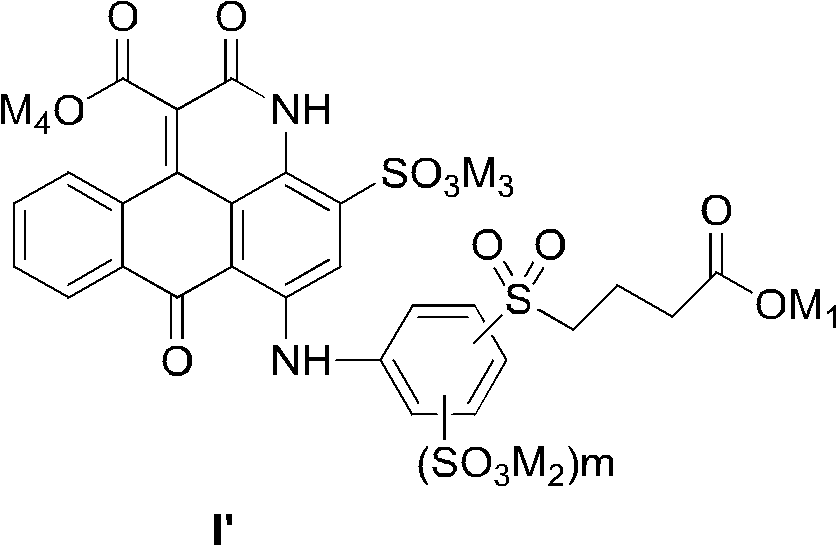
[0071] The preparation of general formula (I) compound and mixture thereof:
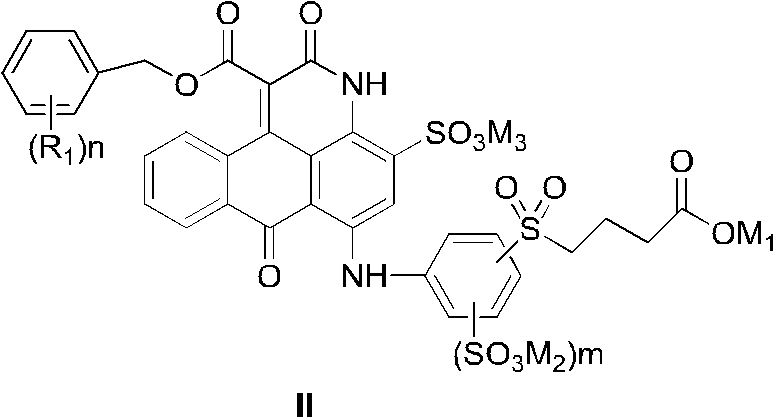
[0072] When preparing the compound of the present invention, different from the prior art using non-dye compounds as starting materials to prepare other anthrapyridone sulfonic acid compounds, the present invention uses low-cost anthraquinone dye derivatives with sulfonic acid groups (III ) or (III') as the basic raw material, in an organic solvent, undergoes a ring-forming reaction with a malonate diester to form an intermediate compound (IV), and then undergoes sulfonation, hydrolysis and benzylation reactions to form a general formula ( I) Compounds, salted out or salt converted to form salts, then isolated and desalted to obtain pure compounds of formula (I).
[0073]
[0074] R in malonate diester and general formula (IV) compound 8 from C 1 -C 4 Alkyl, more preferably methyl and ethyl.
[0075] The synthesis of the compound of general formula (IV) is to make the compound of formula (III)...
Embodiment 1
[0172] (1) Put 100 parts of meta C.I. reactive blue 19, 490 parts of water into the reactor, stir evenly, and then heat to 55°C-60°C. Acid will be generated during the reaction, and the reaction will be promoted by neutralizing the generated acid with a dilute sodium hydroxide solution to keep the pH of the reaction solution at 8.5-9.0. When the pH of the reaction solution remains substantially constant, the reaction is complete. HPLC (high performance liquid chromatography) can also be used to determine if the reaction is complete. After the completion of the reaction, adjust the pH to 9.0 and stir for 1 hour. At this time, a large amount of solids will precipitate, and the precipitated solids are the (III'-RB19) compound. The resulting solid was filtered and dried. Thus, a blue-purple powder of compound (III'-RB19) was obtained in a yield of 83.1 parts. Mass spectrometry: III'-RB19: m / z (-): 483.2 ([ M -H] -1 ). The most abundant and accurate molecular mass of the inte...
Embodiment 2
[0180] Heat the partial aqueous solution of compound Dm1 (containing 9.9 parts of Dm1 dye) obtained in Example 1 to 75°C-80°C, adjust the pH to 9.0, add 9.0 parts of benzyl chloride dropwise within half an hour, and react for 3 hours; then use it for 5 minutes Add 4.5 parts of benzyl chloride dropwise for 2 hours, then add 4.5 parts of benzyl chloride dropwise for 5 minutes, and react for 4 hours. During the whole reaction process, the pH was adjusted to 9.0 with dilute sodium hydroxide solution. After the reaction was completed, 40 parts of sodium chloride was added with stirring to carry out salting out, stirred for 1 hour, and left to stand for 2 hours. The resulting product was filtered and dried, whereby a mixture of sodium salts of the Dm3 compound was obtained as a red powder in a yield of 11.2 parts. The sodium salt mixture has an absorption maximum at 536 nm (in aqueous solution). Mass spectrometry m / z (3-): 259.6 ([ M -3H] -3 ), m / z (2-): 390.2 ([ M -2H] -2 ). ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com