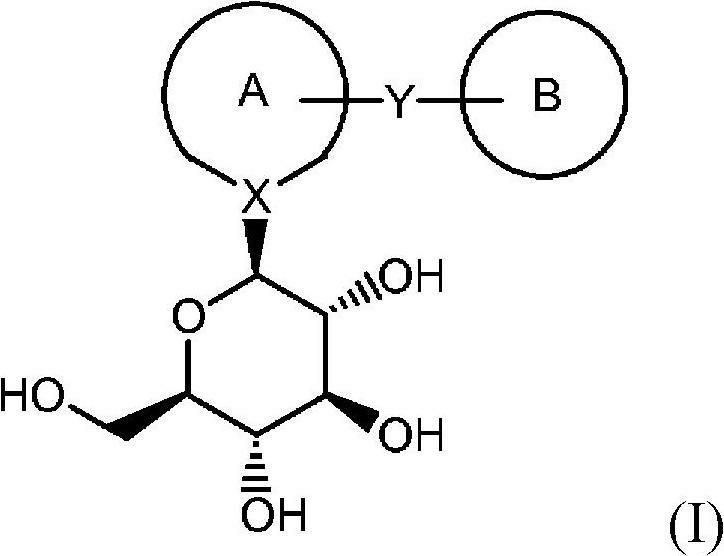
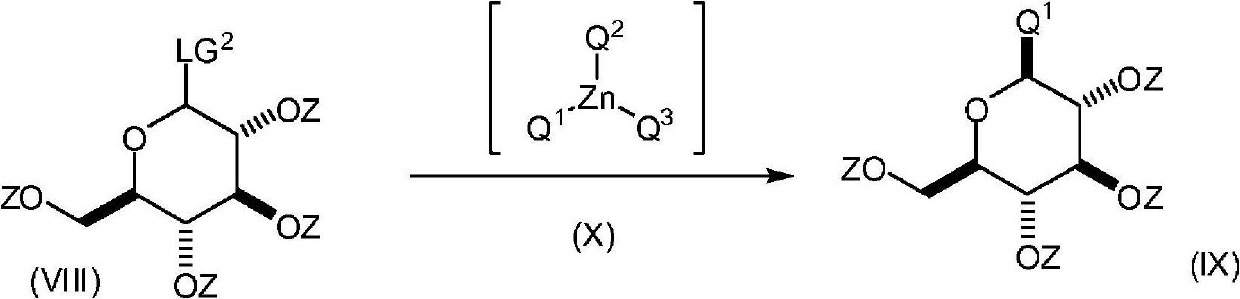
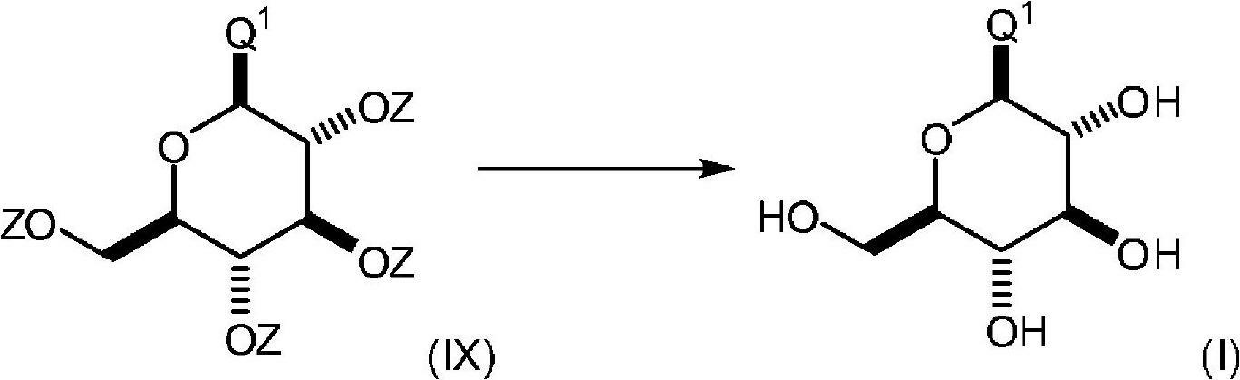
Process for the preparation of compounds useful as inhibitors of SGLT2
一种化合物、溶剂化物的技术,应用在可用作SGLT2的抑制剂的化合物的制备领域,能够解决破坏恶化循环等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0400] Tris(2,2-dimethylpropanoic acid)-(2S,3S,4R,5R,6R)-2-(3-((5-(4-fluorophenyl)thiophene-2- Base) methyl)-4-methylphenyl)-6-(pivaloyloxymethyl)tetrahydro-2H-pyran-3,4,5-triyl ester
[0401]
[0402] In a dry 250 mL round bottom flask with mechanical stirrer under argon atmosphere, 2-(4-fluorophenyl)-5-(5-iodo-2-methylbenzyl)thiophene was dissolved at room temperature (22.20 mmol; 9.06 g) was dissolved in a mixture of dry and degassed toluene (37.00 mL; 32.23 g) / diethyl ether (37.00 mL; 26.24 g). After cooling to -50°C (isopropanol + dry ice bath) with vigorous stirring, (trimethylsilyl)methyllithium (1 M in pentane, 37.00 mL) was added dropwise to the heterogeneous mixture. Thirty minutes after the end of the addition, the conversion was checked by sampling and additional (trimethylsilyl)methyllithium was added as needed. After 15 minutes, zinc dibromide (22.20 mmol; 5.00 g) (ultra dry solid from Aldrich) was added in one portion and the resulting mixture was allowe...
example 2
[0404] Tris(2,2-dimethylpropanoic acid)-(2S,3S,4R,5R,6R)-2-(3-((5-(4-fluorophenyl)thiophene-2- Base) methyl)-4-methylphenyl)-6-(pivaloyloxymethyl)tetrahydro-2H-pyran-3,4,5-triyl ester
[0405]
[0406] In a dry 25mL Schlenk reactor under an argon atmosphere, 2-(4-fluorophenyl)-5-(5-iodo-2-methylbenzyl)thiophene (1.99mmol; 813.71 mg) was dissolved in dry cyclopentyl methyl ether (CPME) (7.2 mL). After cooling to -50°C (acetonitrile+dry ice) under vigorous stirring, n-hexyllithium (2.3M in hexane, 966.31 μL) was added dropwise to the mixture. After 15 minutes, zinc dibromide (996.50 μL; 2M solution in CPME) was added and the resulting mixture was allowed to warm to 15° C. over 1.5 hours. Then 2,3,4,6-O-(2,2-dimethylpropionyl)-α-D-glucopyranose bromide (1.05 g, 1.81 mmol), and the resulting mixture was heated at 85°C overnight. After cooling to room temperature, an aqueous solution of ammonium chloride (1M, 10 mL) and ethyl acetate (15 mL) was added. After stirring for...
example 3
[0408] Tris(2,2-dimethylpropanoic acid)-(2S,3S,4R,5R,6R)-2-(3-((5-(4-fluorophenyl)thiophene-2- Base) methyl)-4-methylphenyl)-6-(pivaloyloxymethyl)tetrahydro-2H-pyran-3,4,5-triyl ester
[0409]
[0410] In a dry 25 mL Schlenk reactor under an argon atmosphere, 2-(4-fluorophenyl)-5-(5-iodo-2-methylbenzyl)thiophene (1.90 mmol; 775 mg ) was dissolved in toluene (3.45 mL) / diethyl ether (3.45 mL). After cooling to -50°C (acetonitrile+dry ice) under vigorous stirring, n-hexyllithium (2.3M in hexane, 920.29 μL) was added dropwise to the mixture. After 15 minutes, zinc dibromide (2.07 mmol; 466 mg) was added in one portion and the resulting mixture was allowed to warm to 15°C over 1.5 hours. The resulting mixture was then cooled to 0° C., and (trimethylsilyl)methyllithium (1M in pentane, 1.9 mL) was added dropwise. After 1 hour, diethyl ether and hexane were evaporated under reduced pressure (400 mmHg) at 15°C. Then, 2,3,4,6-O-(2,2-dimethylpropionyl)-α-D-bromoglucopyranose ( ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com