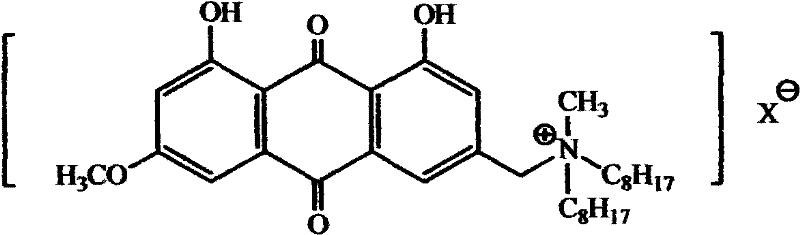
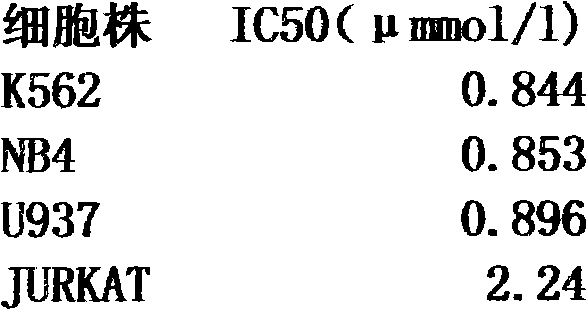Emodin di-n-octyl quaternary ammonium salt with anti-leukemia activity and preparation method thereof
A technology of n-octyl quaternary ammonium salt and methyl di-n-octyl, which is applied in the field of emodin di-n-octyl quaternary ammonium salt and its preparation, can solve the problems of broken cancer cell growth, poor water solubility and high toxicity, and achieve The preparation process is simple, the yield is high, and the preparation conditions are mild
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
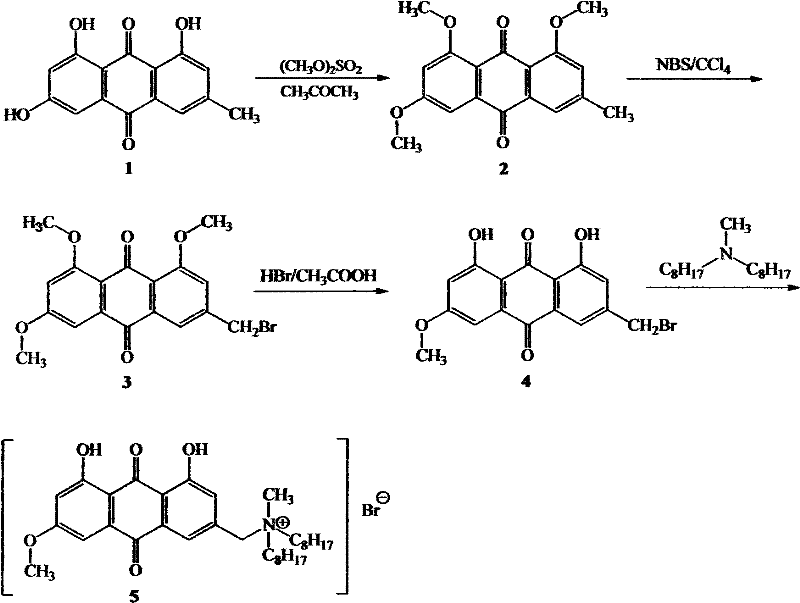
[0018] Embodiment 1: Synthesis of intermediate product 2 (1,3,8-trimethoxy-6-methyl-9,10-anthraquinone)——(a)
[0019] Get 1.6g (5.8mmol) of emodin and dissolve in 120ml of acetone, add 12g (87mmol) of anhydrous potassium carbonate, and slowly add 8ml (87mmol) of (CH 3 O) 2 SO 2 , refluxed for 24h, cooled to room temperature, concentrated solution, added 100ml of water and stirred for 30min, suction filtered, washed with a small amount of cold acetone to obtain the crude product of yellow powder, separated by silica gel column chromatography to obtain 1.52g of bright yellow solid, the silica gel column chromatography The eluent is dichloromethane, and the yield is 82.6%. product structure 1 Confirmed by H NMR, IR, melting point. The characterization data for Intermediate 2 are as follows:
[0020] m.p.226~228℃; IR(KBr)v max / cm-1 : 2941, 2843, 1662, 1601, 1322, 1241, 1022, 759. 1 HNMR (400MHz, CDCl 3 ), δ: 7.65(s, 1H, Ar-H), 7.34(d, 1H, J=2.4Hz, Ar-H), 7.11(s, 1H, Ar-H),...
Embodiment 2
[0021] Example 2: Synthesis of intermediate product 2 (1,3,8-trimethoxy-6-methyl-9,10-anthraquinone)——(b)
[0022] Get 1.6g (5.9mmol) of emodin and dissolve in 200ml of acetone, add 10g (73mmol) of anhydrous potassium carbonate, and slowly add 4ml (43mmol) of (CH 3 O) 2 SO 2 , refluxed for 24h, cooled to room temperature, concentrated solution, added 80ml of water and stirred for 30min, suction filtered, washed with a small amount of cold acetone to obtain a yellow powder crude product, separated by silica gel column chromatography to obtain 1.34g of a bright yellow solid (intermediate product 2), the The eluent of the silica gel column chromatography is dichloromethane, and the yield is 72.8%.
Embodiment 3
[0023] Example 3: Synthesis of intermediate product 2 (1,3,8-trimethoxy-6-methyl-9,10-anthraquinone)——(c)
[0024] Get 1.6g (5.9mmol) emodin and dissolve in 180ml acetone, add 10g (73mmol) anhydrous potassium carbonate, slowly add 4ml (43mmol) of (CH 3 O) 2 SO 2 , refluxed for 20h, cooled to room temperature, concentrated the solution, added 60ml of water and stirred for 30min, suction filtered, washed with a small amount of cold acetone to obtain the crude product of yellow powder, separated by silica gel column chromatography to obtain 1.30g of bright yellow solid (intermediate product 2), the obtained The eluent of the silica gel column chromatography is dichloromethane, and the yield is 70.6%.
[0025] Known by embodiment 1-3, emodin adds anhydrous potassium carbonate and (CH 3 O) 2 SO 2 The chemical reaction can obtain the intermediate product 2 (1,3,8-trimethoxy-6-methyl-9,10-anthraquinone) with a yield of more than 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com