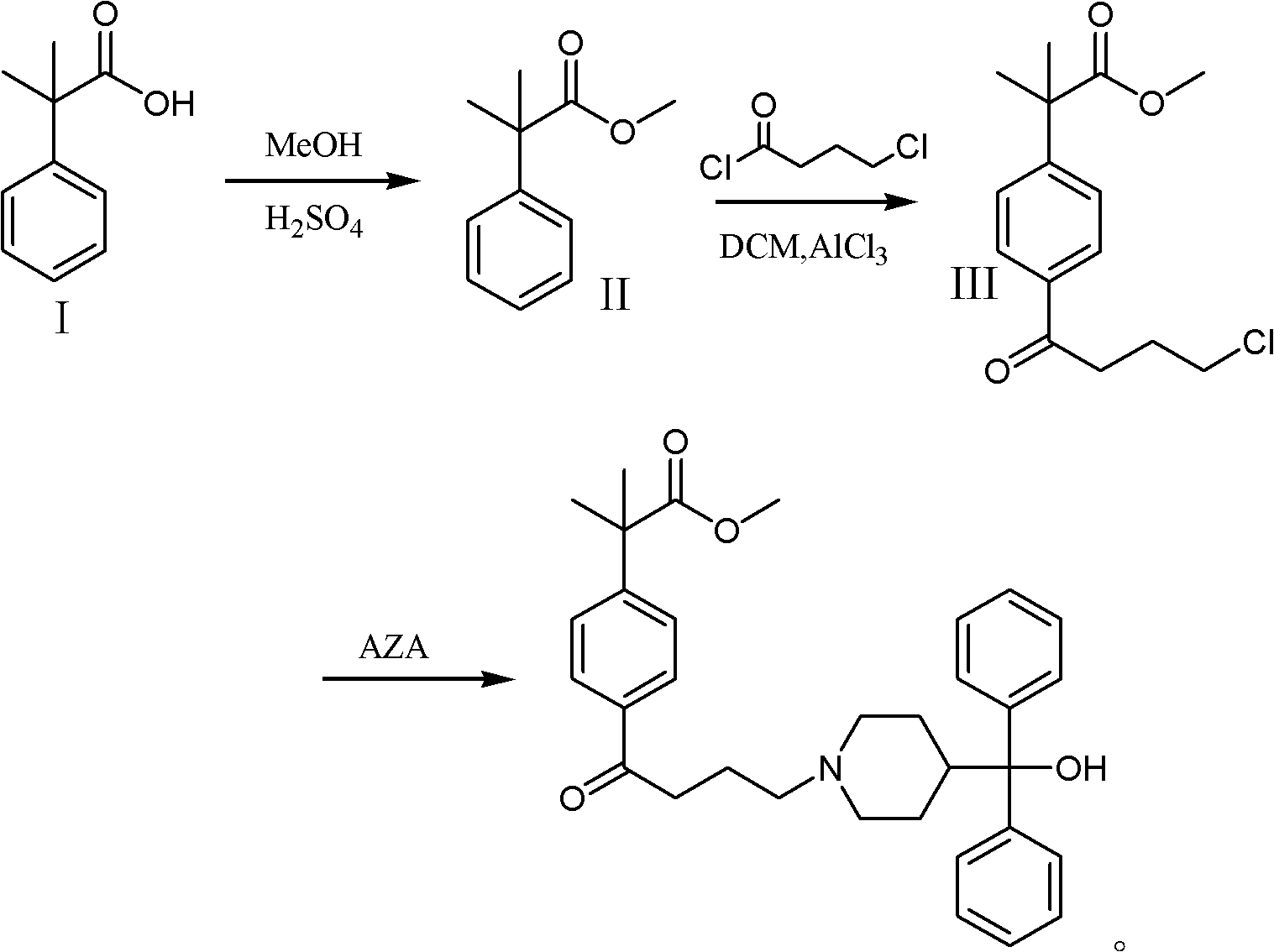
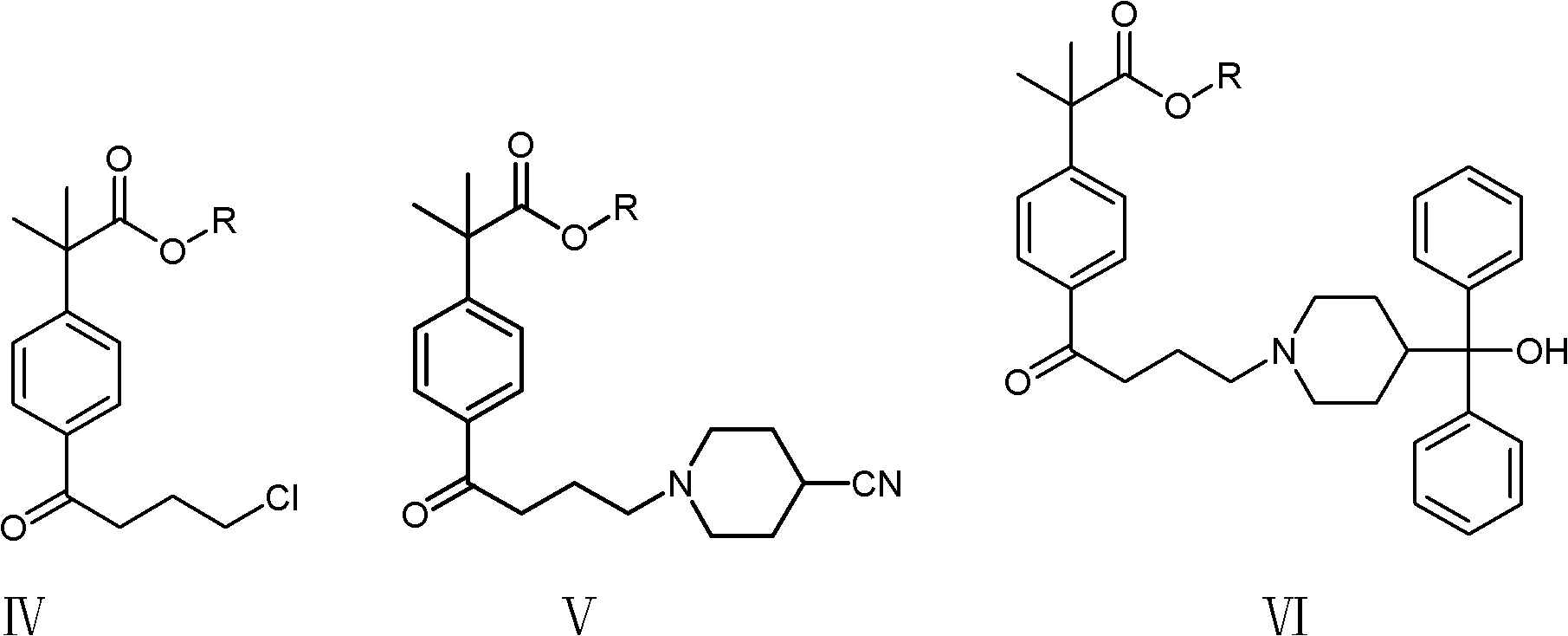
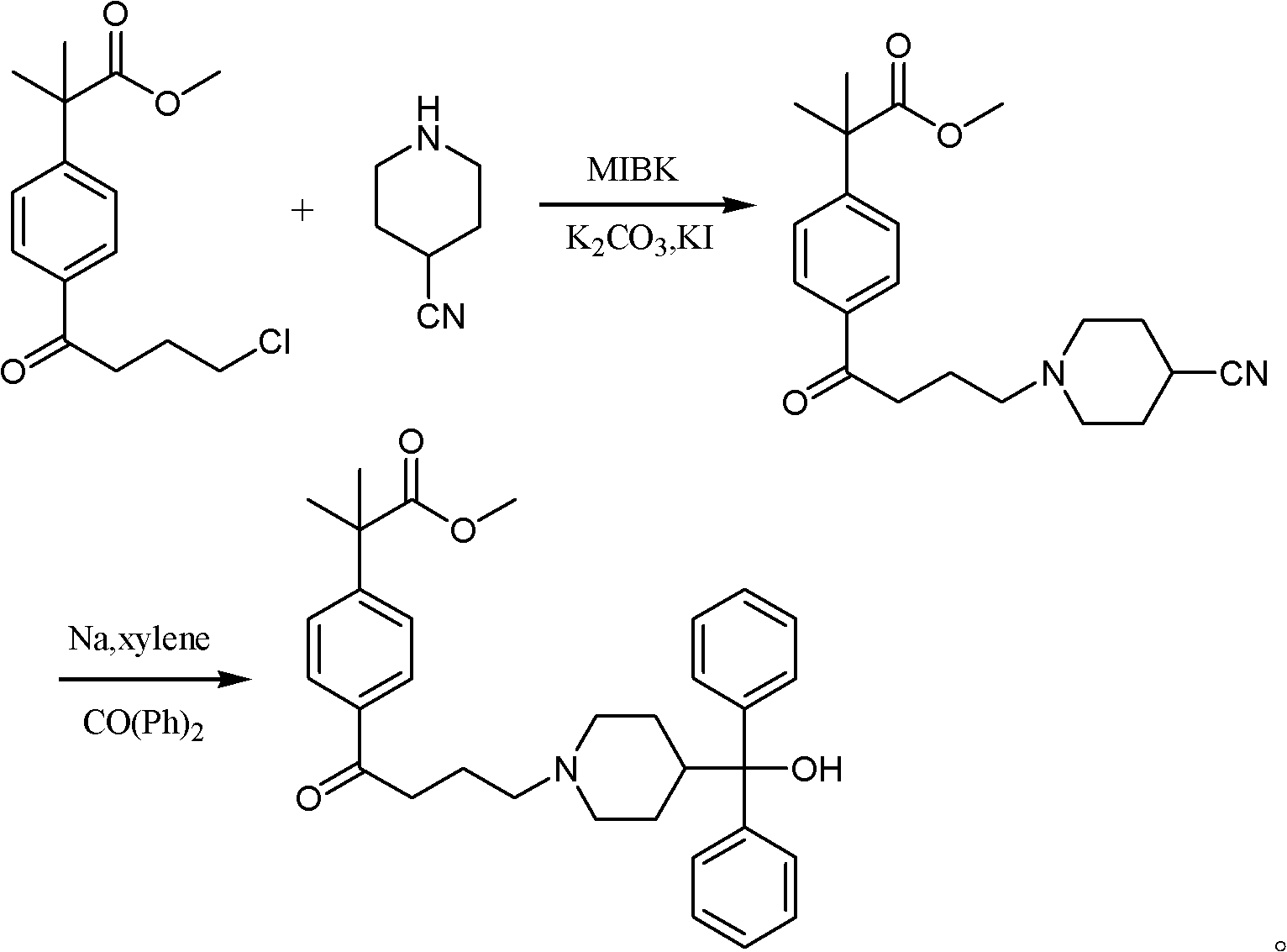
Synthetic method of fexofenadine intermediate
A technology of fexofenadine and a synthesis method, which is applied in the field of organic chemical synthesis, can solve the problems of long reaction time, difficult to remove, and high cost, and achieves the effects of convenient operation, novel reaction route and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Dissolve 28.3 g (0.10 mol) of 4-(4-chlorobutyryl)-α, α-dimethylphenylacetic acid methyl ester in 100 ml of 4-methyl-2-pentanone, stir, and add 4-cyano Base piperidine 11.0g (0.10mol), potassium carbonate 34.5g (0.25mol), catalyst potassium iodide 0.2g, be warming up to 90 ℃, reflux after 4 hours, filter, filter out catalyst and inorganic salt potassium carbonate, and the filtrate is concentrated under reduced pressure to Dry, add 100ml of toluene, 100ml of water, separate the layers, extract the water layer with toluene (50ml*2), combine the organic layers, dry, concentrate to 1 / 3 volume, cool and a white solid precipitates, filter, and dry the solid at 70°C , to obtain 32.1 g of 2-(4-(4-(4-cyano-1-piperidinyl)butyryl)phenyl)-2-methyl-propionic acid methyl ester, yield 90.1%.
[0040] (2) Slowly put 5.0 g (0.22 mol) of sodium metal into 100 ml of xylene in batches, pass through nitrogen protection, heat up to 70 ° C, start stirring, and continuously drop 100 ml of x...
Embodiment 2
[0042] (1) Dissolve 28.3 g (0.1 mol) of 4-(4-chlorobutyryl)-α, α-methyl phenylacetate in 100 ml of toluene, stir, and add 11.0 g (0.1 mol) of 4-cyanopiperidine ), potassium carbonate 34.5g (0.25mol), catalyst potassium iodide 0.2g, be warming up to 90 ℃, after refluxing for 10 hours, filter, filter out catalyst and inorganic salt potassium carbonate, add water 100ml to filtrate, separate layers, water layer and toluene (50ml*2) extraction, combined organic layers, dried, concentrated to 1 / 3 volume, cooled, a white solid precipitated, filtered, and the solid was dried at 70°C to obtain 30.8g 2-(4-(4-(4-cyano Base-1-piperidinyl)butyryl)phenyl)-2-methyl-propionic acid methyl ester, the yield was 86.5%.
[0043] (2) Slowly put 5.0g (0.22mol) of sodium metal into 100ml of xylene in batches, pass through nitrogen protection, heat up to 70°C, start stirring, and continuously drop 100ml of xylene and 16.5g of benzophenone (0.09 mol) clarified solution, dripped in 2 hours. Heat up to...
Embodiment 3
[0045] (1) Except replacing 0.2g potassium iodide with 0.2g sodium iodide, other steps are the same as in Example 1 to obtain 31.0g 2-(4-(4-(4-cyano-1-piperidinyl)butyryl)phenyl )-2-methyl-propionic acid methyl ester, yield 87.0%.
[0046] (2) Except replacing 0.22mol sodium metal with 0.18mol sodium metal, other steps are the same as in Example 1 to obtain 2-[4-[4-[4-(hydroxybenzhydryl)-1-piperidinyl]-1 -Oxobutyl]phenyl]-2,2-dimethyl acetate 39.2g, content 98%, yield 84.9%. The proton nuclear magnetic spectrum data of product and 2-[4-[4-[4-(hydroxybenzhydryl)-1-piperidinyl]-1-oxobutyl]phenyl]-2,2-dimethyl The methyl acetate reference substance is basically the same, and the structure is confirmed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com