Synthesis method of tegoprazan
A synthetic method, Tego's technology, applied in the field of organic synthesis of drugs, can solve the problems of high cost and low yield, and achieve the effects of less by-products, high reaction selectivity, and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
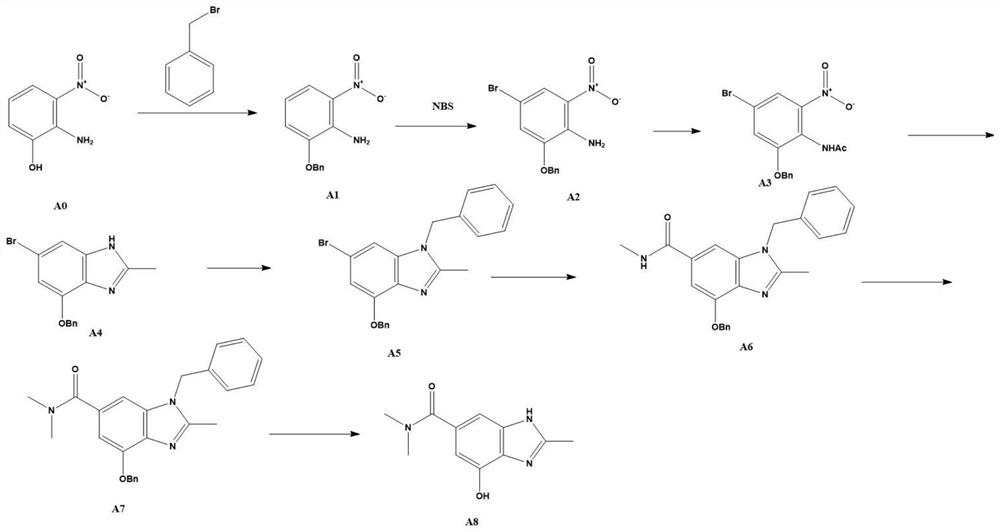
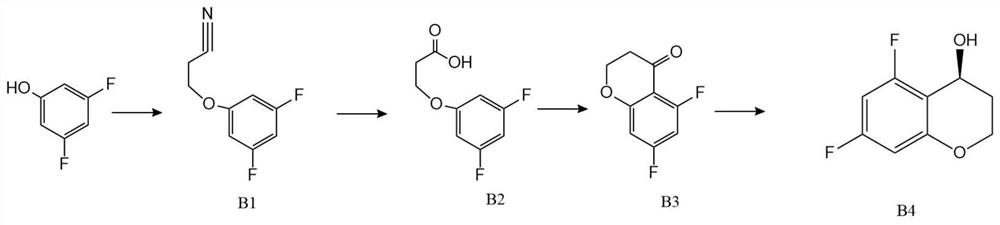
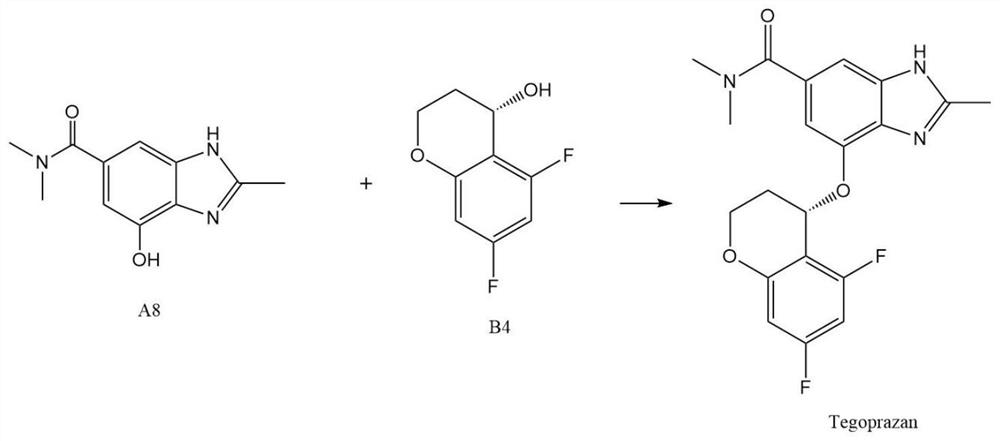
[0047] The present invention proposes a new method for preparing tergrazan, involving 4-hydroxy-N,N,2-trimethyl-1H-benzimidazole-6-carboxamide (stage A), (S)-5 , Preparation of 7-difluoro-3,4-dihydro-2H-chromen-4-ol (stage B), and finally the Mitsunobu reaction.
[0048] The present invention adopts starting material 2-amino-3-nitrophenol to prepare compound A8 through a step-by-step reaction. First, 2-amino-3-nitrophenol and benzyl bromide form ether to prepare compound A1; compound A1 and n -Bromosuccinimide undergoes a bromination reaction to generate compound A2; the amino group of compound A2 reacts with acetic anhydride to generate compound A3; compound A3 undergoes reduction and cyclization with a reducing agent to generate compound A4; after forming a ring, In order to improve the reaction selectivity and reaction yield, the nitrogen of the benzimidazole ring in compound A4 is protected by benzyl bromide or benzyl chloride to generate compound A5; then the aryl bromide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com