Preparation method of 2-bromo-3-methoxypyridine
A technology of methoxypyridine and nitro, which is applied in the field of preparing 2-bromo-3-methoxypyridine, can solve the problems of unsuitability for large-scale industrial production, difficulty in product separation and purification, harsh reaction conditions, etc., and achieve post-processing Easy separation and purification, novel reaction route and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
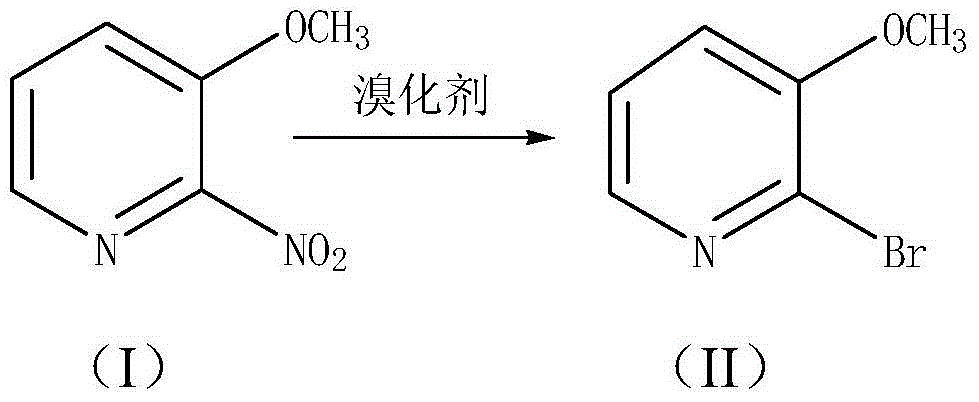
[0022] The synthetic route of the preparation method of the present invention is as follows:
[0023]
[0024] The preparation method of 2-bromo-3-methoxypyridine provided by the present invention will be described in detail below:
[0025] Add 2-nitro-3-methoxypyridine represented by structural formula (I) and an appropriate amount of organic acid solvent into the reaction vessel, stir to dissolve all the 2-nitro-3-methoxypyridine, and wait until the raw materials are completely After dissolving, add brominating agent such as hydrobromic acid, wherein the molar ratio of 2-nitro-3-methoxypyridine to brominating agent is 1:2-3, preferably 1:2-2.5; slowly increase the temperature to 100-140 ℃, reaction for 4-7 hours, preferably reaction temperature of 120-130 ℃, reaction for 5-6 hours. After the reaction is completed, the heating is stopped, and the stirring is continued to cool to room temperature.
[0026] After the reaction solution was distilled under reduced pressure to distill ...
Embodiment 1
[0035] Add 6g of 2-nitro-3-methoxypyridine and 18mL of acetic acid into the reaction flask, stir to dissolve all of them, add 15.8g of hydrobromic acid with a concentration of 40% by mass after the raw materials are completely dissolved, and slowly raise the temperature to 120 ℃, react for 5 hours, stop heating after the reaction is completed, continue to stir to cool to room temperature.
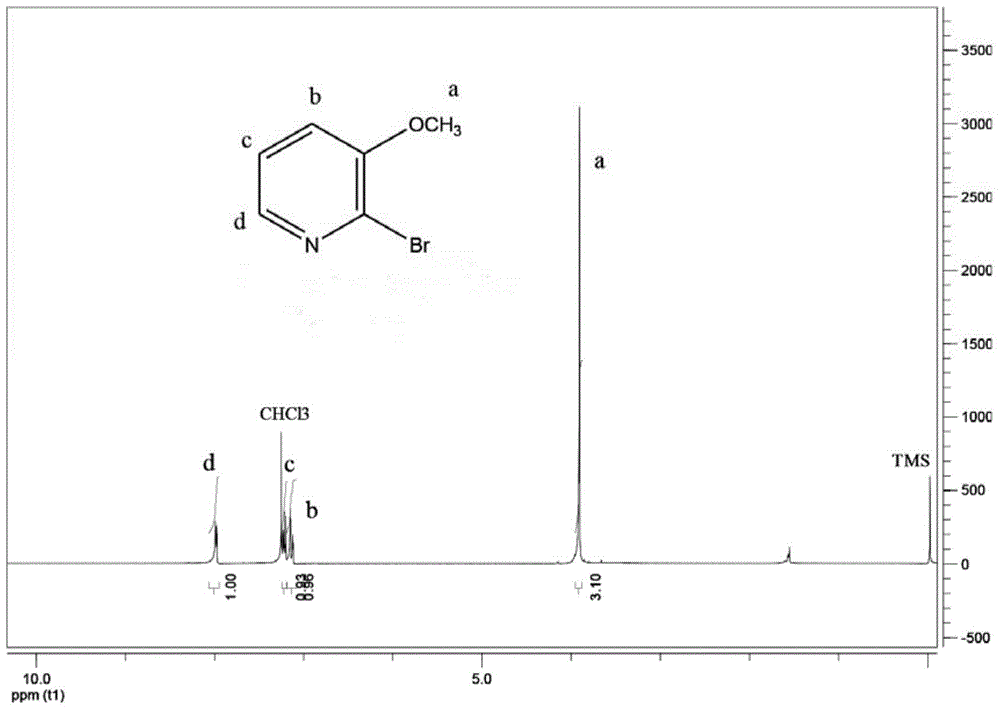
[0036] After the reaction solution was distilled under reduced pressure to distill most of the solvent, the vacuum distillation was stopped. At this time, a yellow solid precipitated out. Filtered. After the filter cake was washed twice with ethyl acetate, the filter cake was dissolved in a small amount of water, and saturated sodium carbonate was added. The pH of the aqueous solution was adjusted to 7-8, a white solid precipitated, filtered, the solid was washed twice with water, and then filtered. After drying, 6.4 g of solid 2-bromo-3-methoxypyridine was obtained. The calculated yield was 88...
Embodiment 2
[0038] Add 10g of 2-nitro-3-methoxypyridine and 30mL of acetic acid into the reaction flask and stir to dissolve them. After the raw materials are completely dissolved, add 28.9g of hydrobromic acid with a concentration of 40% by mass, and slowly increase the temperature to 125 ℃, react for 6h, stop heating after the reaction is completed, continue to stir to cool to room temperature.
[0039] After the reaction solution was distilled under reduced pressure to remove most of the solvent, the vacuum distillation was stopped. At this time, a yellow solid precipitated out. Filtered. After the filter cake was washed twice with ethyl acetate, the filter cake was dissolved in a small amount of water, and saturated hydrogen carbonate was added. Adjust the pH of the sodium aqueous solution to 7-8, a white solid precipitated out, filtered, washed the solid twice with water, and then filtered. After drying, 11.0g of solid 2-bromo-3-methoxypyridine was obtained, and the calculated yield was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com