Modified photoproteins with increased affinity for calcium and enhanced bioluminescence and uses thereof
A light-emitting protein and bioluminescent technology, applied in the direction of animal/human peptides, peptide sources, peptides, etc., can solve the problem of low affinity of aequorin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
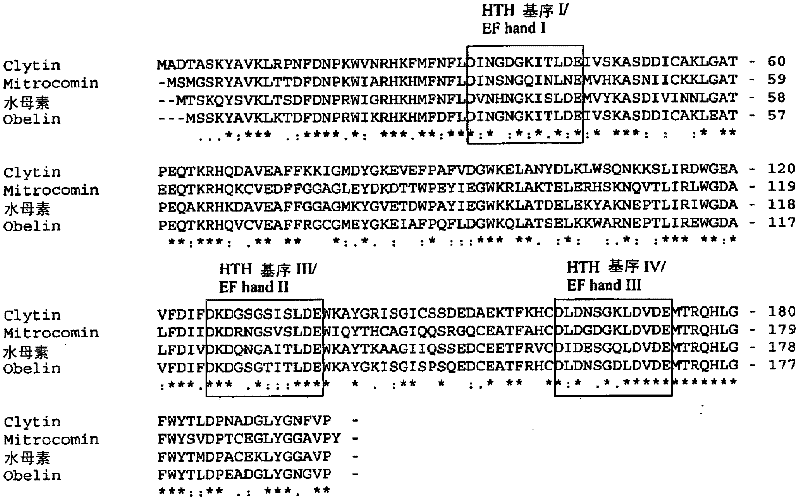
[0095] Example 1: Generation of Clytin variants
[0096] Unmodified forms of Clytin for cytoplasmic expression or forms of Clytin containing mitochondrial targeting sequences were produced. In an exemplary experiment, cDNA encoding unmodified wild-type clytin (termed cyto-clytin-wt) and a clytin with the COX8 mitochondrial leader sequence (termed mt-clytin-wt) were chemically synthesized by GenScript. The cDNA was subsequently subcloned into the mammalian expression vector pcDNA3.1 under the control of the CMV promoter (INVITROGEN). Using the QuikChange kit (STRATAGENE), mutations were introduced into the clytin cDNA by site-directed mutagenesis, including the substitution of lysine at position 168 to aspartic acid (K168D).
[0097] Change the codon AAA encoding the 168th lysine in Clytin to aspartic acid, glutamic acid, asparagine, glycine, glutamine, valine, serine, threonine, tyrosine, arginine A codon for one of amino acid and histidine. Primers used to generate these m...
Embodiment 2
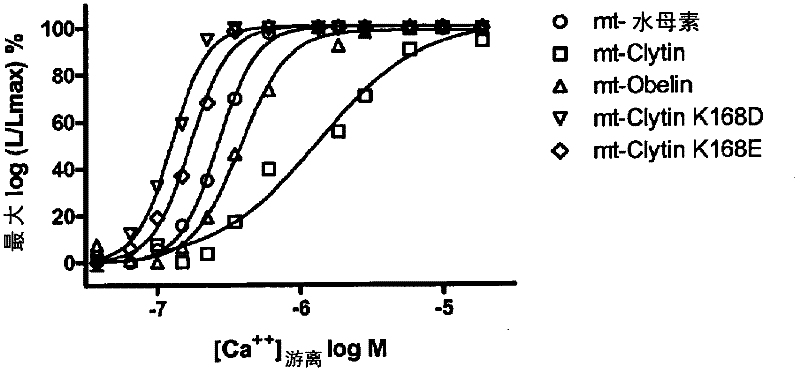
[0098] Example 2: Calcium affinity measurement of wild-type Clytin, modified Clytin K168D and wild-type aequorin
[0099] After production of the modified form of Clytin, the intracellular calcium affinity of the various variants was compared to wt-aequorin in a transient transfection assay using the H1 histamine receptor (Genbank AccessionNo.NM_000861, using H1-specific primers for the brain cDNA library were obtained by PCR, forward primer 5'-GCCGCCACCATGAGCCTCCCCA ATTCCTC-3' (SEQ ID NO: 60), reverse primer 5'-TCATCAGGAGCGAATATGCAG AATTCTC-3' (SEQ ID NO: 61) ) co-transfection.
[0100] In an exemplary experiment, HEK293T cells (ATCC-CRL-11268) were transiently co-transfected using Targetfect-293 reagent (TARGETING SYSTEMS) with 2 μg of each pcDNA3.1-H1 (containing cDNA) and a modified mitochondrial Clytin (called mt-Clytin K168D) that encodes wild-type mitochondrial Clytin (called mt-Clytin) with a lysine at position 168 replaced by an aspartic acid mutation (called mt-Clyt...
Embodiment 3
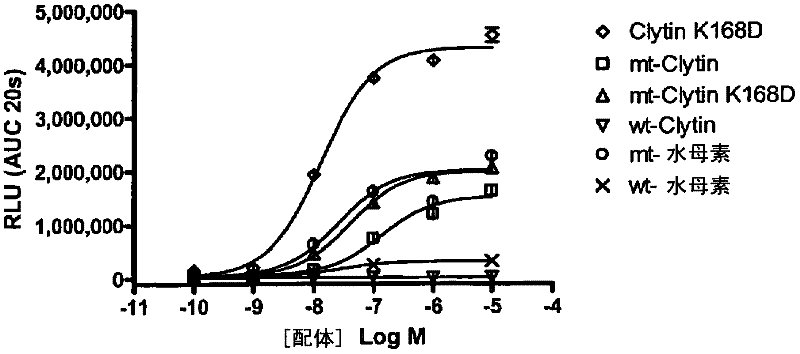
[0102] Example 3: Comparison of GPCR-mediated Luminescence Exhibited by Various Photoprotein Variants
[0103] In another embodiment, the ability of various photoproteins to detect calcium flux in living cells is assessed by adding different concentrations of histamine to the cells to activate the H1 histamine receptor, followed by measuring the bioluminescence of each photoprotein. As described in Example 2, U-2OS cells were transfected with the cDNA encoding the H1 histamine receptor and the cDNA encoding the wild-type photoprotein or the modified photoprotein of the present invention, except that Lipofectamine2000 (INVITROGEN) was used as the transfection agent. dye reagents. On the following day, cells were trypsinized, counted, and plated at a density of approximately 50,000 cells / well in white tissue culture-treated 96-well plates (COSTAR) containing 10% fetal bovine serum. Growth medium consisting of DMEM, non-essential amino acids, HEPES, and penicillin / streptomycin. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com