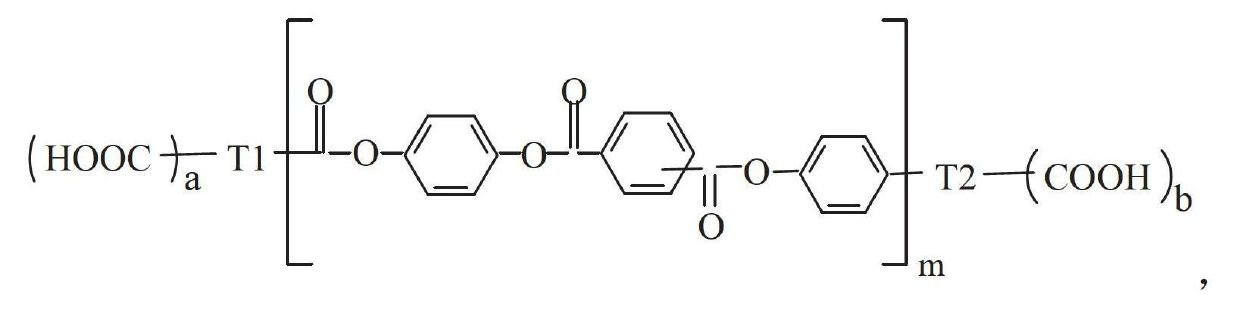
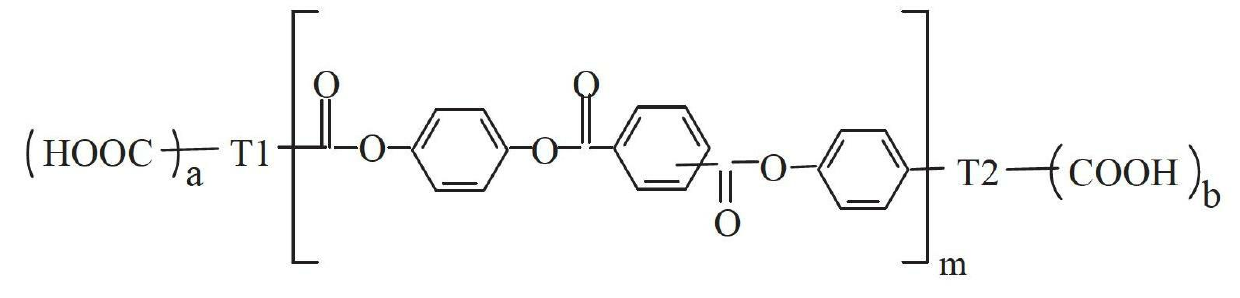
Aryl polyester polyurethane resin
A technology of polyurethane resin and polyester polyurethane, which is applied in the field of aryl polyester polyurethane resin, can solve the problems of migration of small molecule additives and affect the use, etc., and achieve the effect of determining the target molecular structure, simple preparation process, and improved flame retardancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Obtain a kind of aryl polyester polyurethane product APU-1 step by step:
[0035] First, take 4-acetoxybenzoic acid, terephthalic acid and p-diacetoxybenzene, mix them according to the molar ratio of 6:4:3, and synthesize the carboxyl-terminated oligomer C1 according to the method provided in Document 1. The functional group is 2 and the molecular weight is 1942. Secondly, take the oligomer C1 with a molar ratio of 1:2 and diethylene glycol for polycondensation reaction to obtain an aryl polyester polyol with 2 functional groups. Again, polycondensation of aryl polyester polyol and toluene diisocyanate to obtain polyurethane prepolymer. Finally, the polyurethane prepolymer was chain-extended with 1,4-butanediol (the molar ratio of NCO to hydroxyl was 1:1) to obtain polyurethane APU-1.
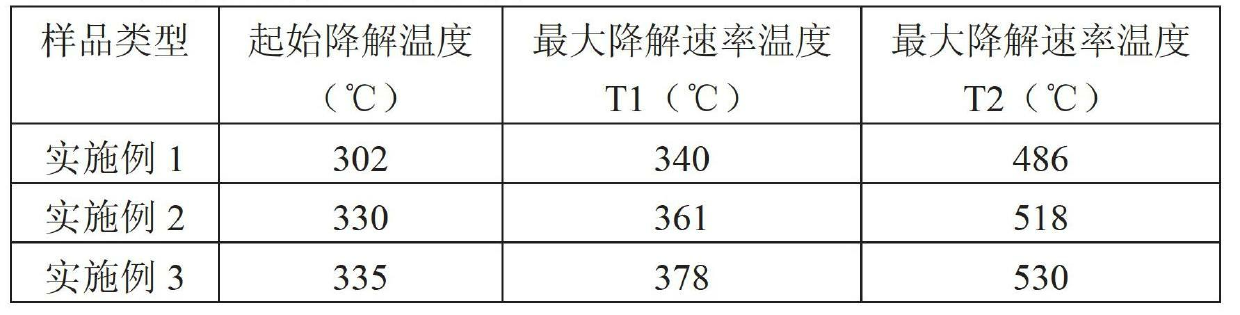
[0036] The thermogravimetric analysis results of polyurethane resin APU-1 are shown in Table 1. The data show that the prepared aryl polyester polyurethane resin has good high temperat...
Embodiment 2
[0038] Polyurethane APU-2 was prepared by reacting the polyurethane prepolymer prepared in Example 1 with the crosslinking agent trimethylolpropane (TMP) (the molar ratio of NCO to hydroxyl was 1:1). The thermogravimetric analysis results of polyurethane resin APU-2 are shown in Table 1. The data show that the prepared aryl polyester polyurethane resin has good high temperature resistance, and this resin can be used as a matrix for high temperature solid powder coatings.
Embodiment 3
[0040] Obtain a kind of aryl polyester polyurethane product APU-3 step by step:
[0041] First, take 1,3,5-benzenetricarboxylic acid, 4-acetoxybenzoic acid, isophthalic acid and p-diacetoxybenzene and mix them in a molar ratio of 2:5:1:2. Methods, synthesis of carboxyl-terminated oligomer C2, its functional group is 4, molecular weight is 1334. In addition, 4-acetoxybenzoic acid, isophthalic acid and p-diacetoxybenzene were mixed according to the molar ratio of 6:3:4, and the acyl-terminated oligomer A1 was synthesized according to the method provided in Document 1. Its functional group is 2 and its molecular weight is 1778. Secondly, the carboxyl-terminated oligomer C2 and the acyl-terminated oligomer A1 are mixed, and the polycondensation reaction obtains a body-shaped carboxyl-terminated aryl polyester; the carboxyl-terminated aryl polyester continues to react with excess ethylene glycol to obtain Aryl polyester polyol. Finally, a thermosetting polyurethane APU-3 was obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com