Method for etching and preparing ultra-hydrophobic aluminum surface by using saline solution containing copper ions and chloride ions
A technology of chloride ion and salt solution, which is applied to the device for coating liquid on the surface, special surface, coating, etc. It can solve the problems of complex equipment and technology, dangerous chemical reagents, low processing efficiency, etc., and achieve stable hydrophobic performance, Easy operation and high processing efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
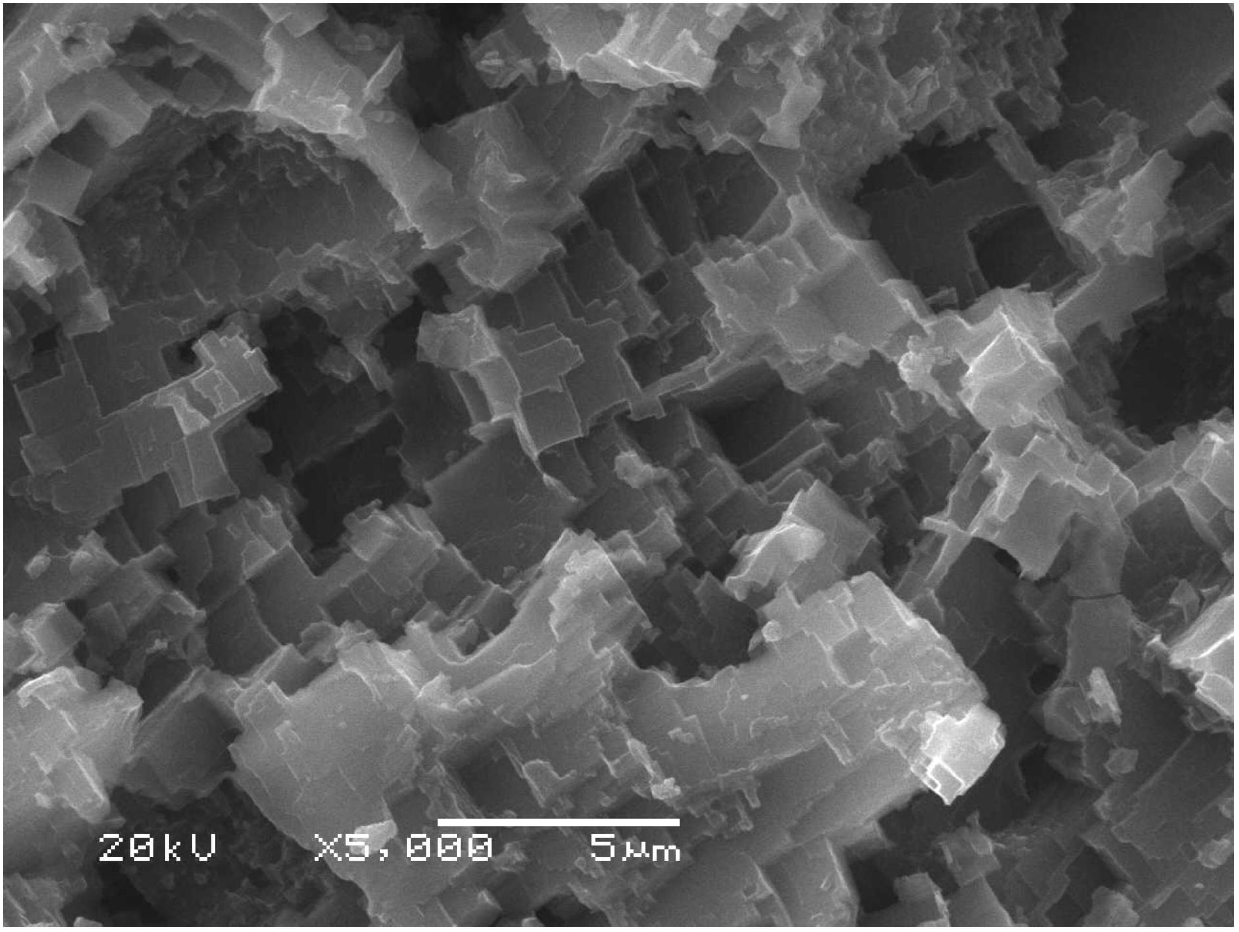
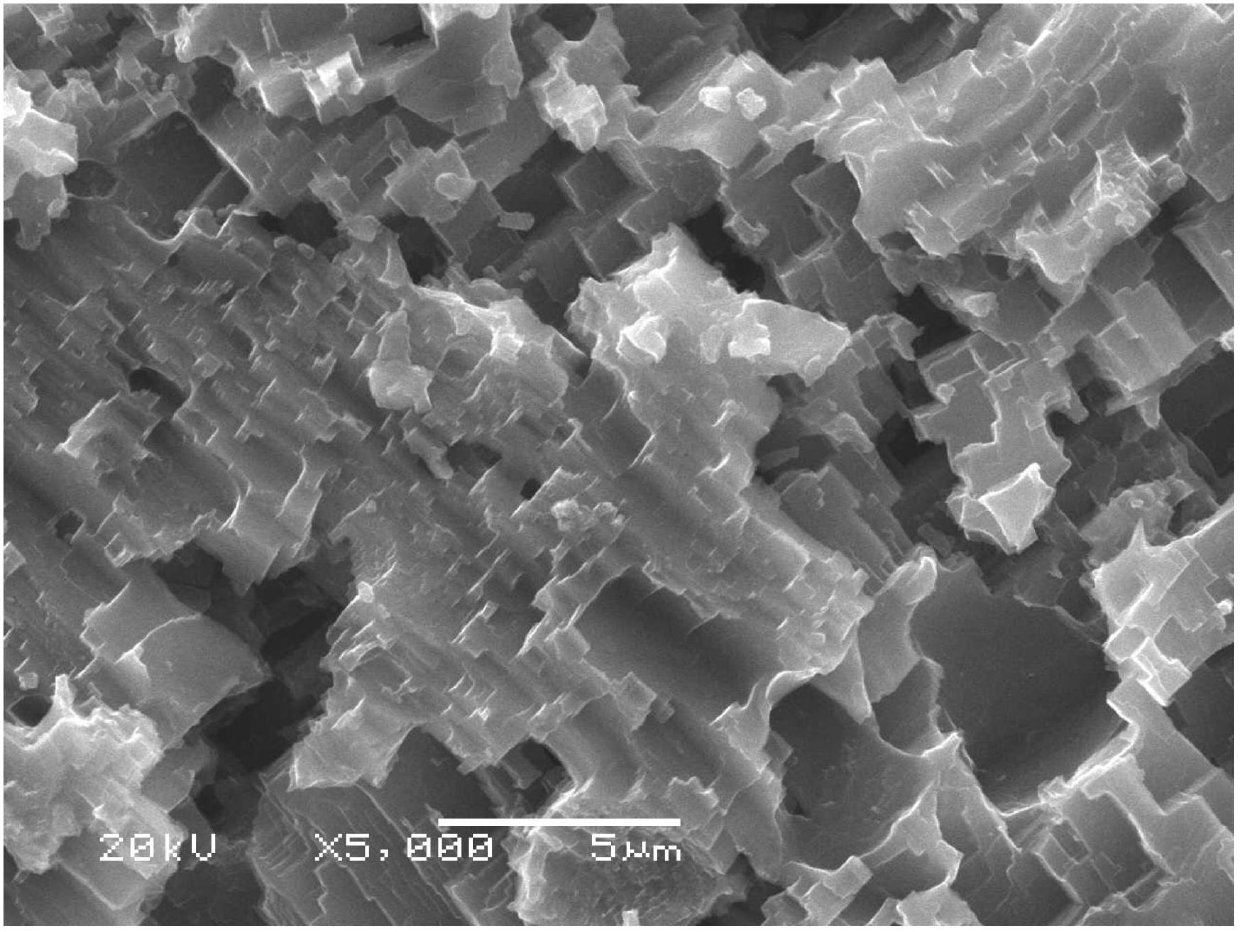
[0022] The 30 × 40 × 2 mm aluminum plate was polished with 1200# and 1500# sandpaper to remove the oxide layer and impurities on the surface. The polished aluminum plate was ultrasonically cleaned with absolute ethanol and deionized water in sequence, and dried. Then immerse the cleaned aluminum plate in an aqueous solution containing 1 mol / L copper chloride for 10 s, the copper ions and aluminum on the surface of the aluminum plate undergo a substitution reaction, the aluminum is corroded into aluminum ions, and the copper ions are reduced to copper and deposited on the aluminum plate. On the surface, after the aluminum plate is taken out, the deposited copper is cleaned with an ultrasonic cleaner and dried. Finally, soak the dried aluminum plate in a 1% fluorosilane ethanol solution for 1 h, take it out and put it in an oven at 100 °C for 10 min. Scanning electron microscopy showed that the surface of superhydrophobic aluminum had a rough structure composed of micro-nano-sc...
Embodiment 2
[0024] The 30 × 40 × 2 mm aluminum plate was polished with 1200# and 1500# sandpaper to remove the oxide layer and impurities on the surface. The polished aluminum plate was ultrasonically cleaned with absolute ethanol and deionized water in sequence, and dried. Then soak the cleaned aluminum plate in an aqueous solution containing 1 mol / L copper sulfate and 2 mol / L sodium chloride for 30 s. On the surface of the aluminum plate, the copper ions and aluminum undergo substitution reactions, and the aluminum corrodes into aluminum ions, and the copper ions It is reduced to copper and deposited on the aluminum surface. After the aluminum plate is taken out, the deposited copper is cleaned with an ultrasonic cleaner and dried. Finally, soak the dried aluminum plate in a 1% fluorosilane ethanol solution for 1 h, take it out and put it in an oven at 100 °C for 10 min. Scanning electron microscopy showed that the surface of superhydrophobic aluminum had a rough structure composed of ...
Embodiment 3
[0026] The 30 × 40 × 2 mm aluminum plate was polished with 1200# and 1500# sandpaper to remove the oxide layer and impurities on the surface. The polished aluminum plate was ultrasonically cleaned with absolute ethanol and deionized water in sequence, and dried. Then soak the cleaned aluminum plate in an aqueous solution containing 1 mol / L copper nitrate and 2 mol / L sodium chloride for 35 s. On the surface of the aluminum plate, copper ions and aluminum undergo substitution reactions, aluminum corrodes into aluminum ions, and copper ions are reduced to Copper is formed and deposited on the aluminum surface. After the aluminum plate is taken out, the deposited copper is cleaned with an ultrasonic cleaner and dried. Finally, soak the dried aluminum plate in a 1% fluorosilane ethanol solution for 1 h, take it out and put it in an oven at 100 °C for 10 min. Scanning electron microscopy showed that the surface of superhydrophobic aluminum had a rough structure composed of micro-na...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com