Process for preparing ethanol from synthesis gas via methyl alcohol
A technology for synthesis gas and ethanol production, which is applied in the preparation of hydroxyl compounds, the preparation of organic compounds, the production of bulk chemicals, etc., can solve the problems of high transportation cost, high cost of cellulase, and high cost, and achieves high process energy utilization rate , The effect of high ethanol yield and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
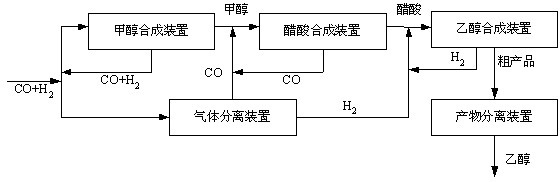
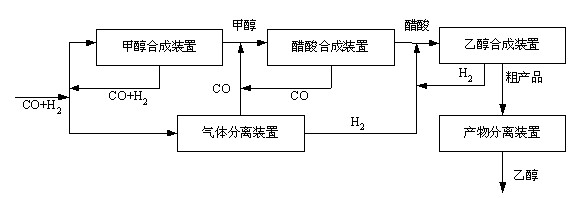
[0024] 480,000 tons / year ethanol synthesis (based on 300 days of operation per year)
[0025] Will H 2 :CO=2:1. The output is 2.46 million m 3 The synthesis gas per day is divided into two parts, the first part is 1.23 million m 3 / Day for methanol synthesis, the second part of 1.23 million m 3 / Day for CO and H 2 Separated and used as raw material for acetic acid synthesis and ethanol synthesis. The method disclosed in patent CN102133498A is used for cryogenic separation of CO and H in synthesis gas 2 , The output of CO after separation is 1.07 million m 3 / Day, H 2 The output is 160,000 m 3 / day.
[0026] Methanol synthesis adopts Lurgi tubular isothermal synthesis tower low pressure synthesis process. The raw material synthesis gas volume is 1.23 million m 3 / Day, the gas entering the tower after heat exchange has a temperature of 225°C, directly enters the reaction tube of the synthesis tower from the upper part, and reacts to form methanol under the action of the catalyst....
Embodiment 2
[0030] 280,000 tons / year ethanol synthesis (based on 300 days of operation per year)
[0031] Will H 2 :CO=2:1. The output is 1.48 million m 3 The synthesis gas per day is divided into two parts, the first part is 740,000 m 3 / Day for methanol synthesis, the second part is 740,000 m 3 / Day for CO and H 2 Separated and used as raw material for acetic acid synthesis and ethanol synthesis. Use the method disclosed in patent CN102191086A to separate CO and H in synthesis gas 2 , The output of CO after separation is 640,000 m 3 / Day, H 2 The output is 100,000 m 3 / day.
[0032] Methanol synthesis adopts ICI multi-stage cold shock tower low pressure synthesis process, the reaction tower is divided into 4 beds, and the catalyst adopts ICI51-7 type catalyst. The raw material synthesis gas volume is 980,000 m 3 / Day, the temperature of the gas entering the tower after the heat exchange and part of the cold shock gas is mixed, and the temperature is 220°C. It enters the first stage bed from ...
Embodiment 3
[0036] 360,000 tons / year ethanol synthesis (calculated based on 300 days of operation per year)
[0037] Will H 2 :CO=2:1. The output is 1.85 million m 3 The synthesis gas per day is divided into two parts, the first part is 925,000 m 3 / Day for methanol synthesis, the second part of 925,000 m 3 / Day for CO and H 2 Separated and used as raw material for acetic acid synthesis and ethanol synthesis. The method disclosed in patent CN102133498A is used for cryogenic separation of CO and H in synthesis gas 2 , The output of CO after separation is 805,000 m 3 / Day, H 2 The output is 120,000 m 3 / day.
[0038] Methanol synthesis adopts Linde spiral coiled tube isothermal synthesis tower methanol synthesis process. The raw material synthesis gas volume is 925,000 m 3 / Day, the gas entering the tower after heat exchange has a temperature of 225°C, directly enters the reaction tube of the synthesis tower from the upper part, and reacts to form methanol under the action of the catalyst. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com