Method for recovering raw materials in preparation process of 7-phenylacetamide-3-chloromethyl-3-cephem-4-carboxylic acid p-methoxybenzyl
A technology of chloromethyl cephalosporanic acid and p-methoxybenzyl ester, which is applied in the field of raw material recovery, can solve problems such as waste and environmental pollution, achieve the effects of reducing environmental pressure, simple operation, and easy large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
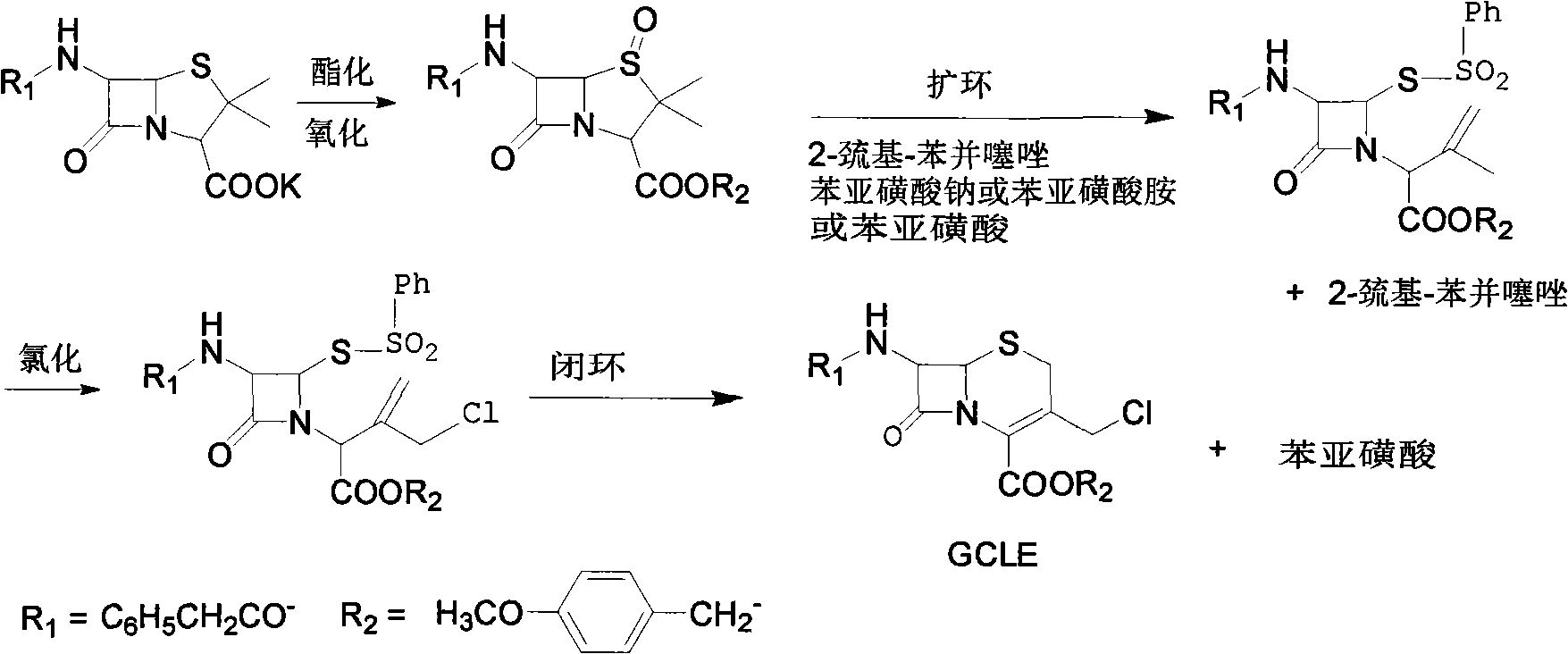
[0028] A: Take 200g of penicillin sulfoxide, add 1000ml of dioxane and 1000ml of toluene as solvent, 100ml of isopropanol (or ethanol), add 77g of 2-mercaptobenzothiazole at 100°C, after 3 hours of reaction, reduce the pressure to 0.1atm, remove dioxane and toluene, add 1000ml isopropanol to dissolve, add dropwise the pre-dissolved benzenesulfinic acid isopropanol (or ethanol) solution at 35°C, the solution contains 77g sodium benzenesulfinate, drop Add for 2-3 hours, cool down to 0°C, keep warm for 2 hours, filter, wash with isopropanol, and dry to obtain the intermediate of azetidinone sulfinic acid; collect the isopropanol washing solution to obtain the post-treatment alcohol solution;
[0029] Add ethyl acetate corresponding to 50% volume of the alcohol solution under stirring to the alcohol solution obtained after the post-treatment in the above reaction, cool to 0-5°C, a white solid precipitates, filter the solid, and distill the filtrate to remove the solvent, then add ...
Embodiment 2
[0033] A. Take 150g of penicillin sulfoxide ester, add 1100ml of dioxane and 1100ml of toluene each as a solvent, 110ml of isopropanol, add 85g of 2-mercaptobenzothiazole at 90°C, complete the reaction in 4 hours, reduce the pressure to 0.1atm, remove Dioxane and toluene, add 900ml of isopropanol to dissolve, add dropwise the pre-dissolved isopropanol solution of benzenesulfinate at 35°C, the solution contains 90g of sodium benzenesulfinate, add dropwise for 3h, cool down to 0°C, Insulate for 2 hours, filter, wash with isopropanol, and dry to obtain the intermediate of azetidinone sulfinic acid; collect the isopropanol washing solution to obtain the post-treatment alcohol solution;
[0034]Slowly add the alcohol solution obtained after the post-treatment in the above reaction into 8 times the volume of ice water, and a bright yellow solid is precipitated, filtered, dried, and recrystallized with a mixture of 20 parts by volume of petroleum ether and ethyl acetate, of which petr...
Embodiment 3
[0038] A. Take 250g of penicillin sulfoxide, add 900ml of dioxane and 900ml of toluene each as a solvent, 85ml of isopropanol, add 90g of 2-mercaptobenzothiazole at 110°C, and complete the reaction in 3 hours. Reduce the pressure to 0.1atm, remove Dioxane and toluene, add 900ml of isopropanol to dissolve, add dropwise the pre-dissolved benzenesulfinic acid isopropanol solution at 35°C, the solution contains 85g of sodium benzenesulfinate, add dropwise for 2h, cool down to 0°C, Insulate for 2 hours, filter, wash with isopropanol, and dry to obtain the intermediate of azetidinone sulfinic acid; collect the isopropanol washing solution to obtain the post-treatment alcohol solution;
[0039] The alcohol solution obtained after the post-treatment in the above reaction is evaporated to remove the solvent, then added petroleum ether to make a slurry, dispersed and solidified, filtered, and recrystallized with a mixture of 15 to 25 times petroleum ether and ethyl acetate, wherein petro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com