Spinning dope and method for producing biomedical fiber
A kind of spinning stock solution and biological technology, applied in spinning solution preparation, wet spinning method, medical science, etc., can solve problems such as complex manufacturing procedures and difficult control of HA content of chitosan fibers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1.1 Preparation of biomedical fibers containing HA and chitosan
[0028] 50g of chitosan, 25g of acetic acid (CH 3 COOH) was mixed with 950 g of water and stirred at room temperature for 3 hours to completely dissolve the chitosan. Then, 25 g of HA was added to form a spinning dope. In addition, 50 g of sodium hydroxide, 475 g of methanol, and 475 g of water were mixed to prepare a molding liquid.
[0029] In the process of wet spinning, the pump flow rate of the wet spinning machine is set to 1.5ml / min, and the spinning dope is squeezed into the forming liquid through a spinning nozzle (with 500 holes, each hole has a diameter of 10 μm) Form biomedical fibers. The biomedical fibers were then placed in a vacuum dryer to remove residual solvents (water and methanol).
[0030] 1.2 Preparation of biomedical fibers containing gelatin and chitosan
[0031] In this example, biomedical fibers were prepared using the same method as in Example 1.1, except that gelatin was u...
Embodiment 2
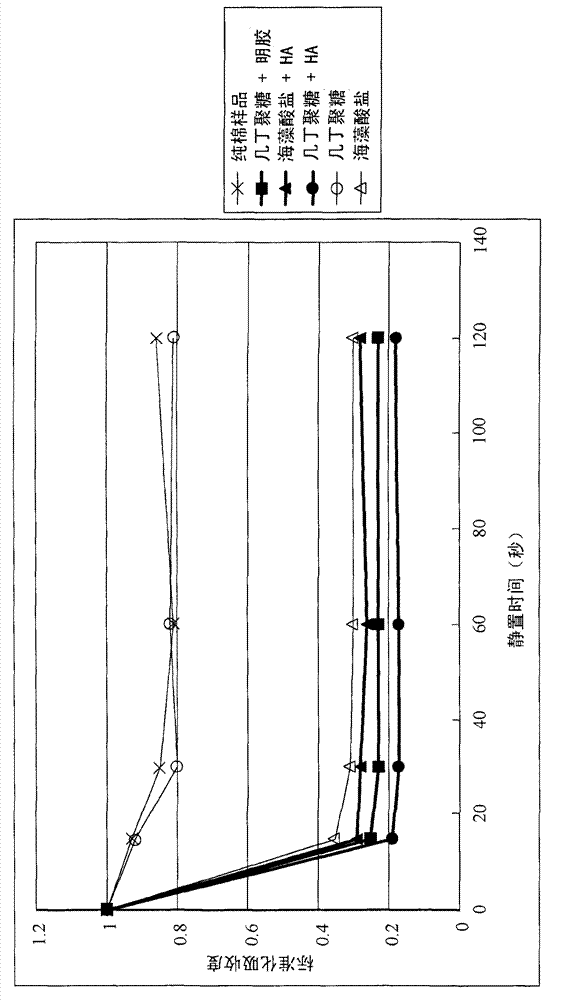
[0035] Embodiment 2: Characteristic analysis of the biomedical fiber of embodiment 1
[0036] The blood-clotting effects of the biomedical fibers prepared in Example 1 were quantified by the following test methods. Briefly, 100 μL of blood was dropped on 0.1 g of biomedical fabric samples (nonwoven fabrics made of the biomedical fibers of Examples 1.1, 1.2 and 1.3). After standing for a period of time (for example, 15, 30, 60 and 120 seconds), the biomedical material fabric sample with blood was soaked in a container containing 10 ml of physiological saline, and shaken for 4 minutes. Uncoagulated blood on biomedical fabric samples will dissolve in saline, while coagulated blood will still adhere to the fabric samples. Then the biomedical material fabric samples were taken out from the saline solution, and the blood content in the saline solution was analyzed by enzyme-linked immunosorbent assay (ELISA). Absorbance at 540nm. The lower the absorbance, the lower the concentrat...
Embodiment 3
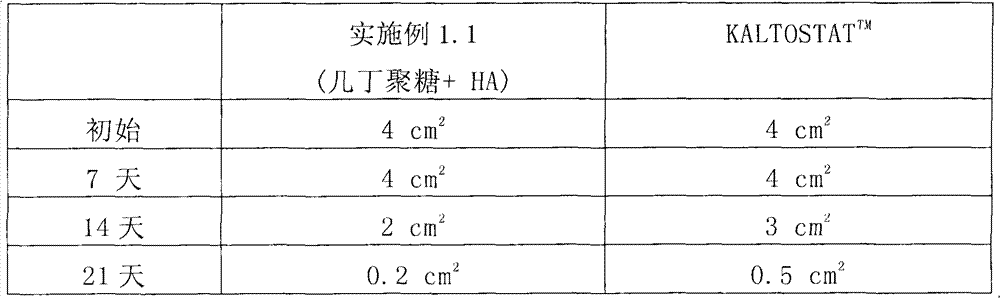
[0041] Embodiment 3: the healing-promoting effect of the biomedical fiber of embodiment 1
[0042] The healing-promoting effect of the biomedical fiber of Example 1.1 was quantified by the following tests. Briefly, two wounds with the same area of 4 cm were formed on the skin of an ICR mouse. 2 . Then, one of the wounds was treated with the non-woven fabric made in Example 1.1 and covered the wound, while the other wound was treated with the commercial product KALTOSTAT TM Wounds were treated and covered as a control group. After a period of time, such as 7 days, 14 days, and 21 days, the areas of the two wounds were measured respectively, and the measurement results are listed in Table 1.
[0043] Table I
[0044]
[0045] The biomedical fiber of Example 1.1 exhibits an excellent healing-promoting effect. As shown in Table 1, after 14 days of treatment, the area of the wound treated by the nonwoven fabric made in Example 1.1 was 2 cm 2 , while KALTOSTAT TM The a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com