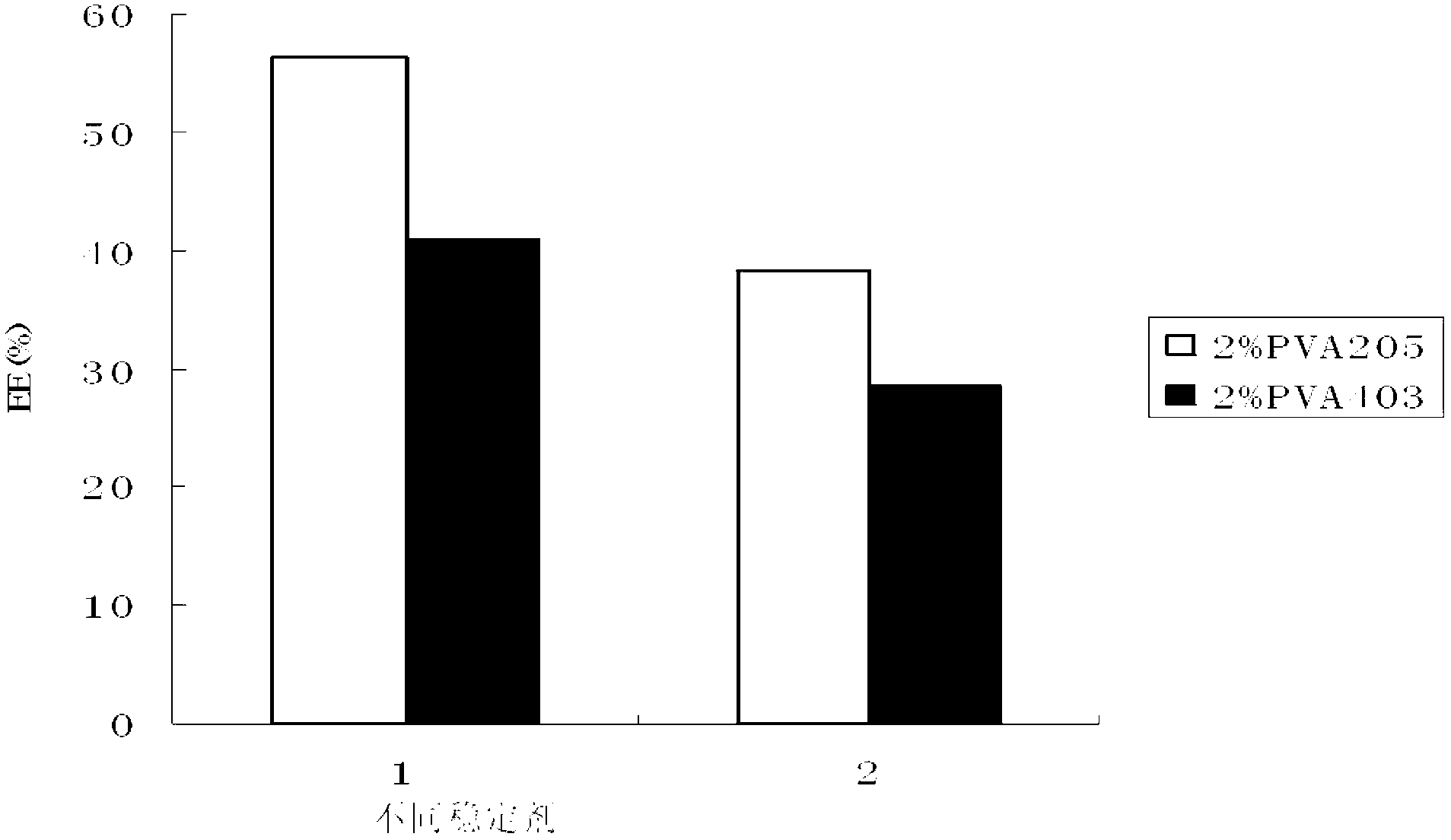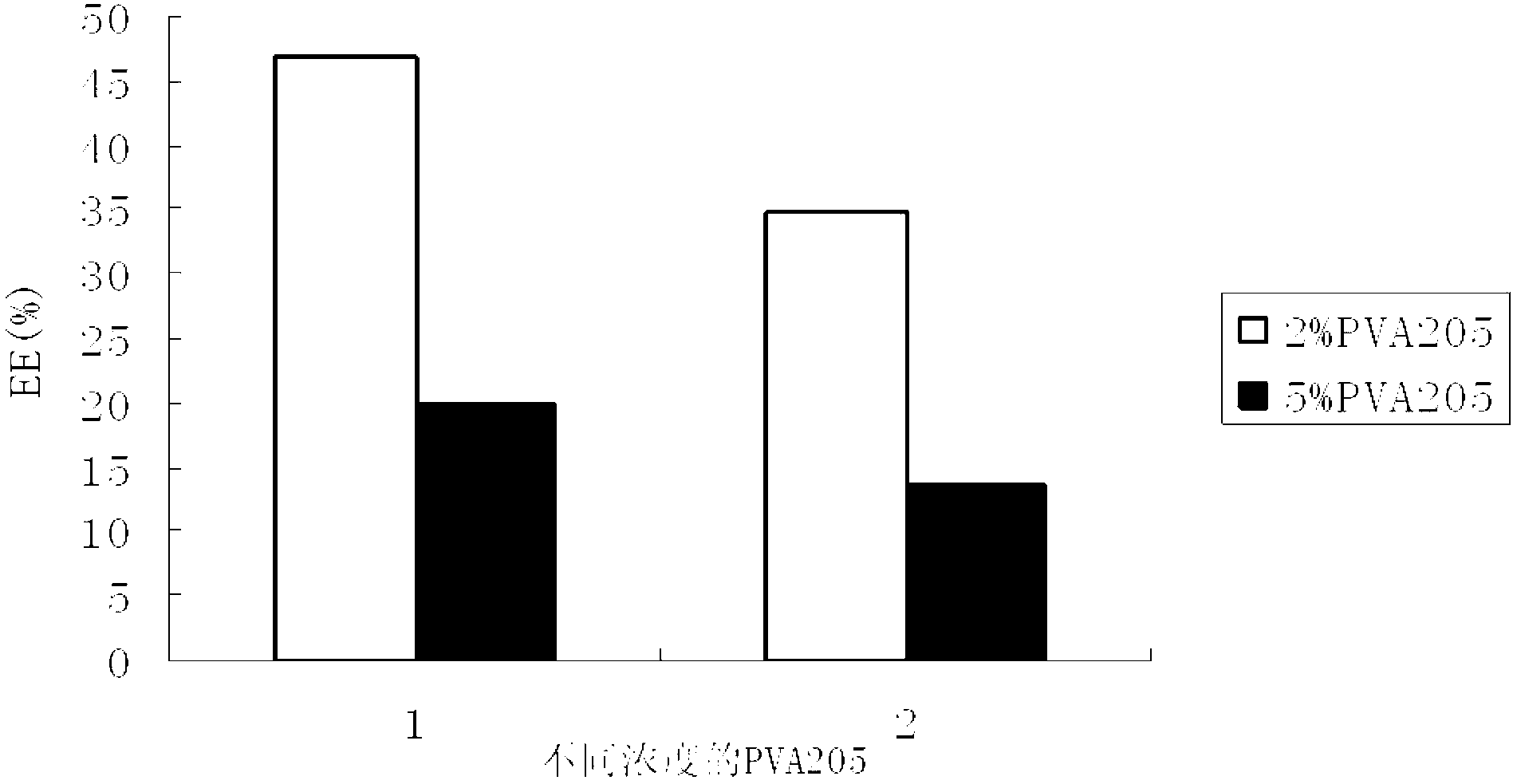Anti-tumor combined medicament
An anti-tumor and drug technology, applied in the directions of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problem of the combined use of epirubicin and quercetin that is not reported in the literature, and achieve the goal of reducing toxicity and reversing drug resistance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
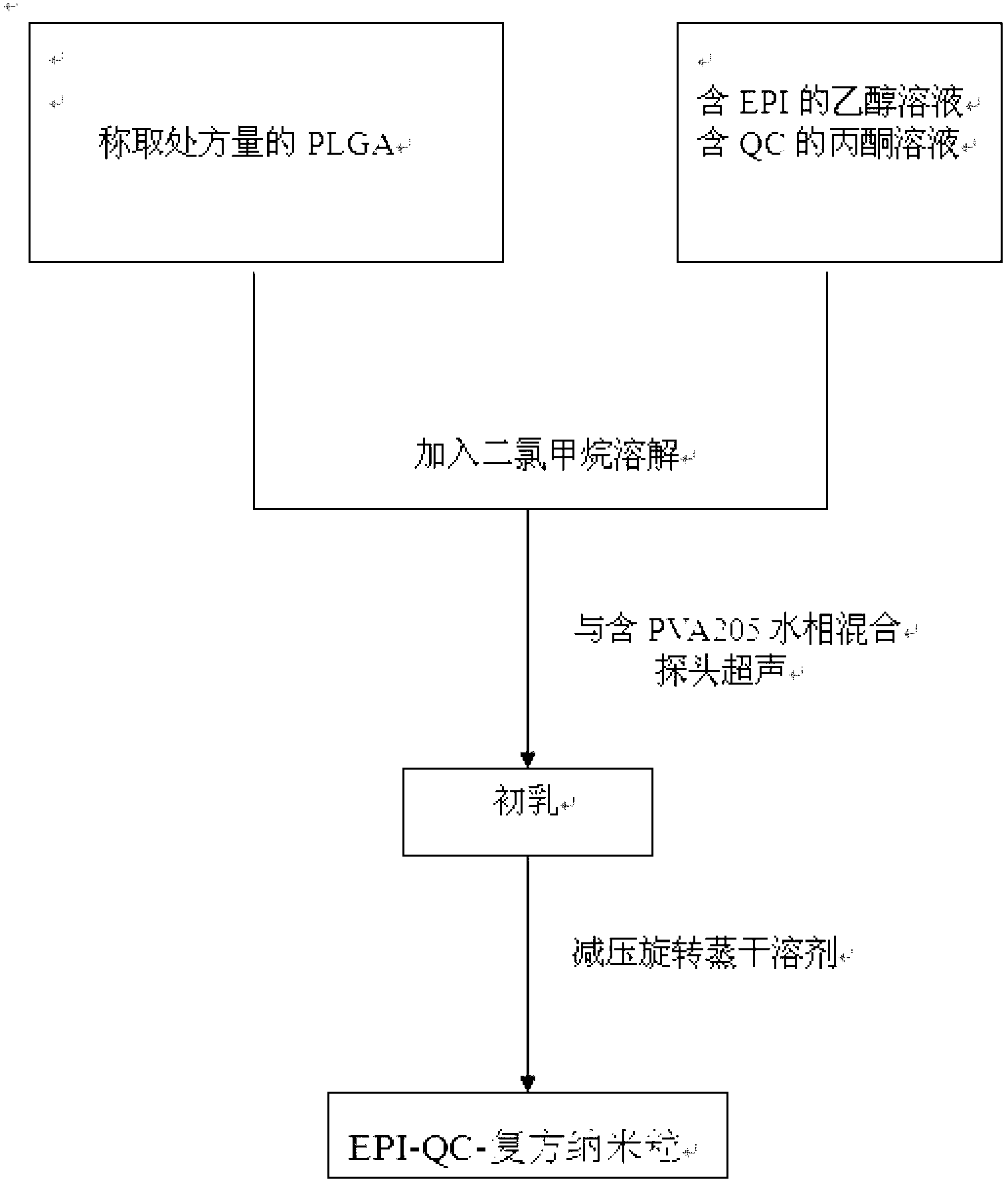
[0047] Embodiment 1 The preparation of drug freeze-dried preparation of the present invention
[0048] Prescription: epirubicin 0.2g, quercetin 2g, PLGA100g, PVA2055g, mannitol 50g; 1000 tubes in total;
[0049] The specific steps are:
[0050] a. Prepare the EPI solution with a concentration of 1mg / ml with ethanol, and prepare the QC solution with a concentration of 10mg / ml in acetone for subsequent use;
[0051] b. Weigh PLGA, add dichloromethane and acetone solution to dissolve PLGA, then add ethanol solution to form the organic phase, the volume ratio of dichloromethane: acetone: ethanol is: 1: 0.4: 0.1, mix well, and mix with 2% PVA The water phase is mixed, and the 200W power probe is ultrasonicated for 3 minutes, and then placed in a round bottom flask. Under the conditions of 25 ° C and 50 r / min, the organic solvent is removed on a rotary evaporator under reduced pressure, and nanoparticles (EPI-QC compound nanoparticles) are obtained after 40 minutes. Add distilled ...
Embodiment 2
[0053] Example 2: Research on the Preparation Technology of Nanoparticles Simultaneously Encapsulating Epirubicin Hydrochloride and Quercetin
[0054] In terms of nanoparticle wrapping materials, the present invention selects the polylactic acid copolymer (PLGA) which has been approved abroad and has good biocompatibility and degradability. Circulation is transformed into water and carbon dioxide to be excreted from the body, non-irritating and non-toxic.
[0055] The selected model drugs of the present invention, epirubicin and quercetin, are quite different in their physical and chemical properties. The former is a water-soluble drug, while the latter is a water-insoluble and fat-insoluble drug, and there are great difficulties in the preparation process.
[0056] 1 Instruments and reagents
[0057] 1.1 Instrument
[0058]
[0059] 1.2 Reagents
[0060] PLGA Shandong Medical Device Research Institute
[0061] PVA Kuraray International Trading (Shanghai) Co., Ltd.
[...
Embodiment 3
[0146] Embodiment 3 EPI-QC compound nanoparticle quality research of the present invention and its freeze-drying process research
[0147] The morphology, particle size distribution and Zeta potential of the final formulation of EPI-QC compound nanoparticles were investigated, and the freeze-drying process was studied.
[0148] 1 Instruments and reagents
[0149] 1.1 Instrument
[0150] Malvern Zetasizer Nano ZS90 laser particle size analyzer Malvern UK
[0151] pHS-4C acidity meter Chengdu Ark Technology Development Co., Ltd.
[0152] ModulyoD freeze dryer American thermoelectric company
[0153] 1.2 Reagents
[0154] Epirubicin hydrochloride Jinan Weidu Chemical Co., Ltd.
[0155] Quercetin Gift from Chengdu Superman Phytochemical Co., Ltd.
[0156] EPI-QC compound nanoparticles were prepared according to the method of Example 1
[0157] Acetonitrile is chromatographically pure; other reagents are analytically pure.
[0158] 2 Experimental methods
[0159] 2.1 Appea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com