Organic heteropoly hybrid catalyst for esterification reaction and preparation method thereof
A technology for esterification reaction and heteropoly acid, which is applied in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., to achieve convenient recovery, simple synthesis process and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
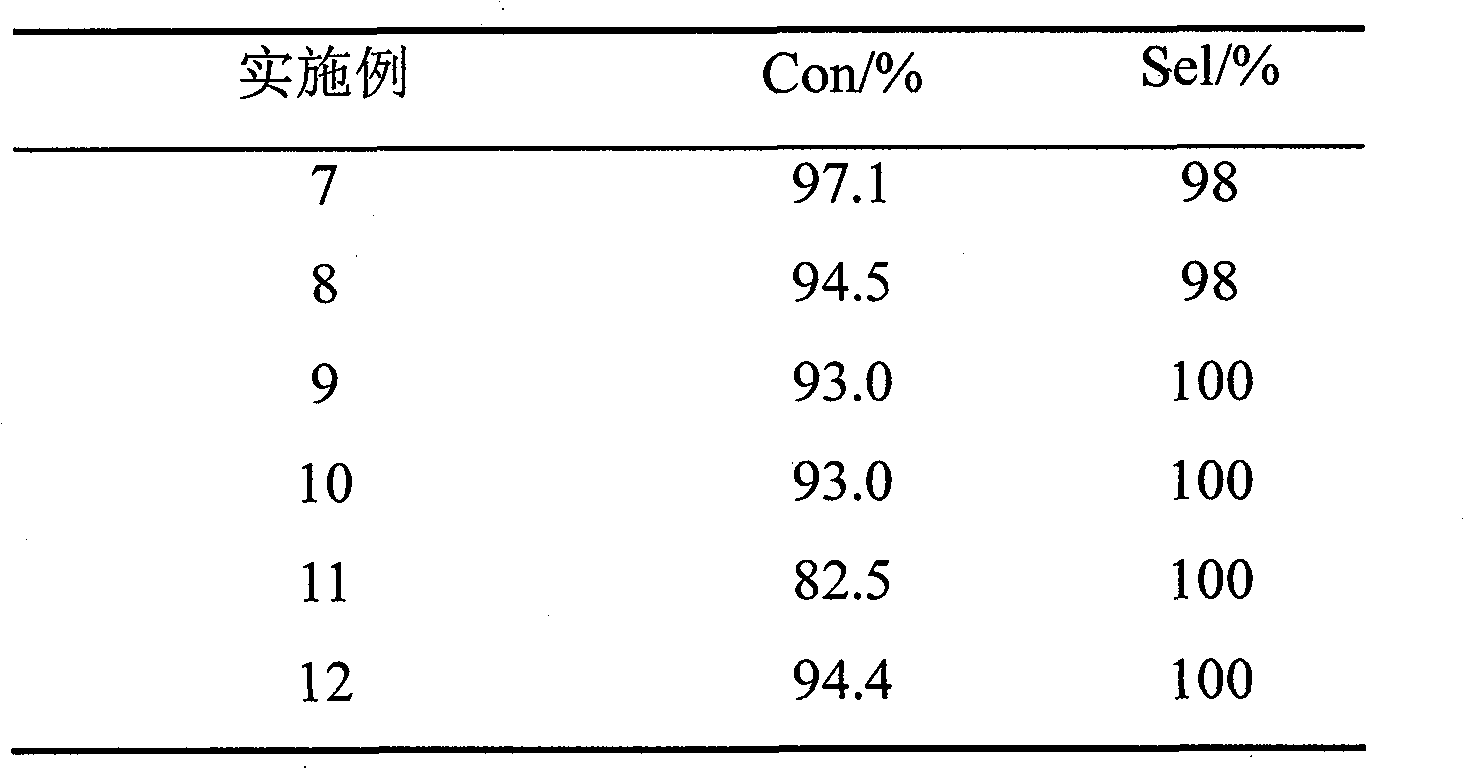
Examples
Embodiment 1
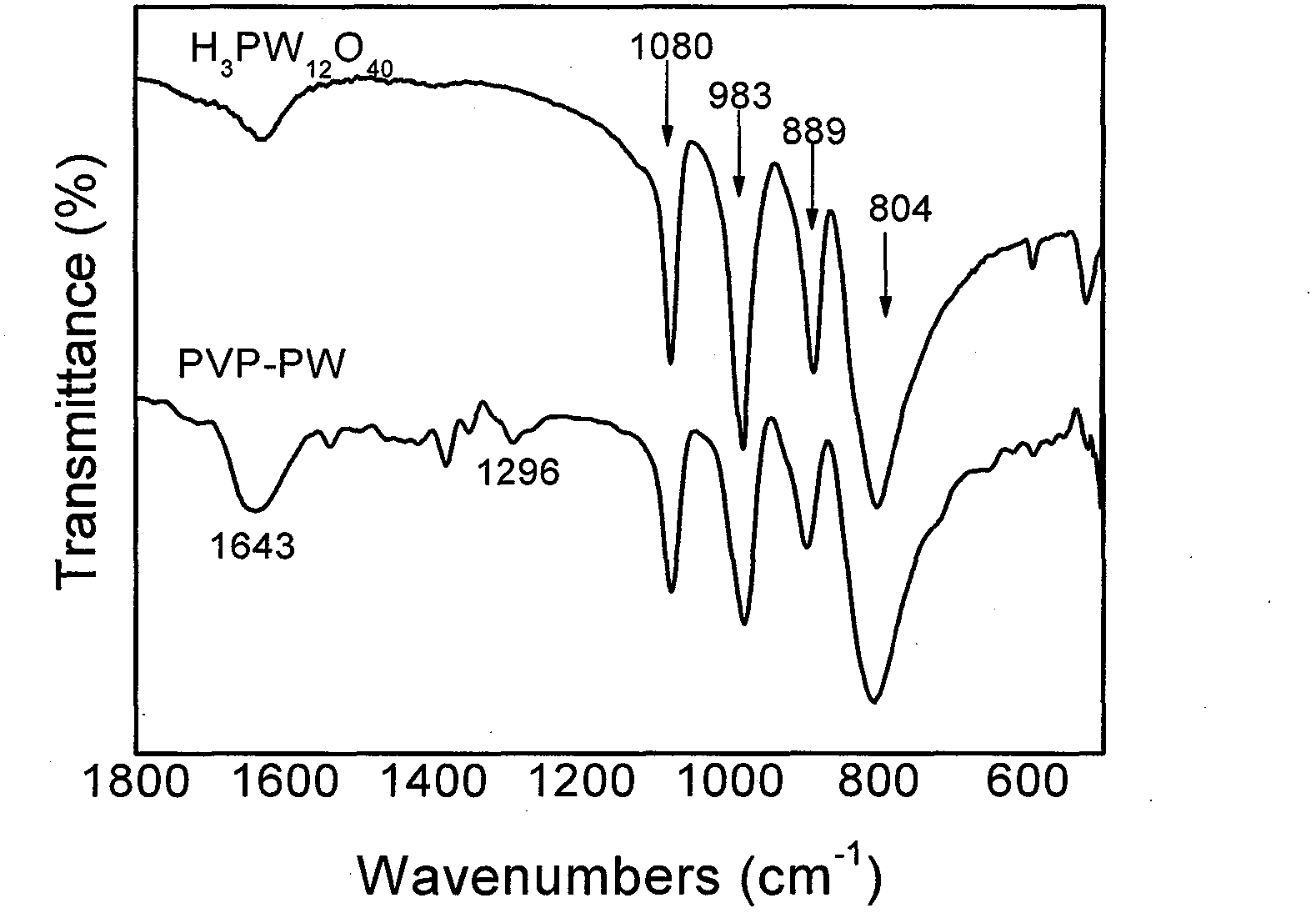
[0018] Add 60mL of distilled water, 0.58g of polyvinylpyrrolidone and 5.0g of 12-phosphotungstic acid into a 100mL round bottom flask, and stir at 50°C for 12h. The solvent was removed by rotary evaporation, washed repeatedly with ethanol, and dried at 100° C. for 12 hours to obtain 5.3 g of polyvinylpyrrolidone-phosphotungstic acid hybrid catalyst (PVP-PW). IR (KBr, v / cm -1 )( figure 1 ) characterization results are: 1643, 1296, 1080, 983, 889, 804cm -1 .
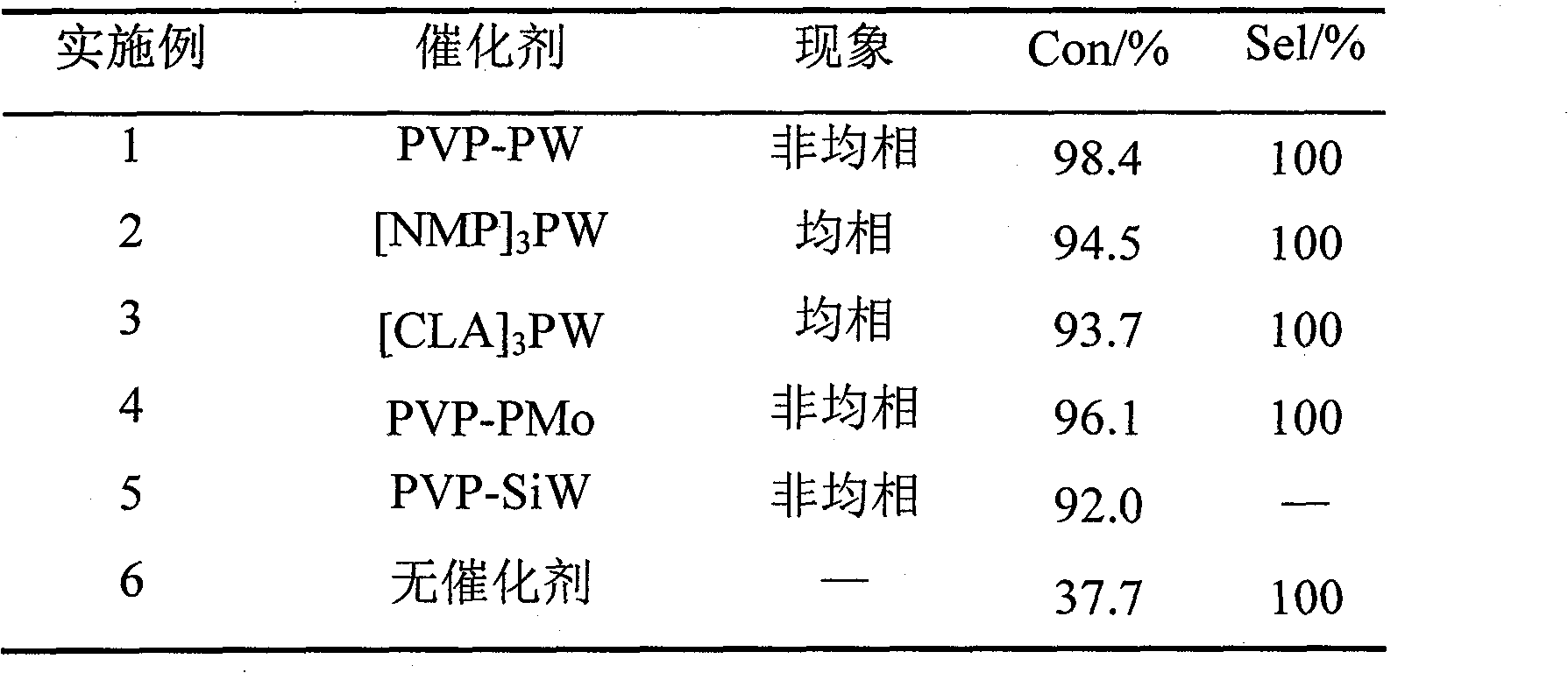
[0019] In a 50mL three-necked flask equipped with a magnetic stirrer, a spherical condenser and a water separator, add 0.2g catalyst PVP-PW, 30mmol acetic acid and 36mmol n-butanol, heat the oil bath to 110°C, and react for 1.5h. The results of the catalytic reactions are shown in Table 1.
Embodiment 2
[0021] Add 60mL of distilled water, 0.52g of methylpyrrolidone and 5.0g of 12-phosphotungstic acid into a 100mL round bottom flask, and stir at 50°C for 12h. The solvent was removed by rotary evaporation, washed repeatedly with ethanol, and dried at 100°C for 12 hours to obtain 5.2 g of methylpyrrolidone-phosphotungstic acid hybrid catalyst ([NMP] 3 PW).
[0022] The resulting catalyst [NMP] 3 PW is used for the esterification reaction of acetic acid and n-butanol, carried out according to the method described in Example 1, and the results of the catalytic reaction are shown in Table 1.
Embodiment 3
[0024] Add 60mL of distilled water, 0.59g of caprolactam and 5.0g of 12-phosphotungstic acid into a 100mL round bottom flask, and stir at 50°C for 12h. The solvent was removed by rotary evaporation, washed repeatedly with ethanol, and dried at 100°C for 12 hours to obtain 5.2 g of caprolactam-phosphotungstic acid hybrid catalyst ([CLA] 3 PW).
[0025] The resulting catalyst [CLA] 3 PW is used for the esterification reaction of acetic acid and n-butanol, carried out according to the method described in Example 1, and the results of the catalytic reaction are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com