Method for preparing CLT acid (6-chloro-3-nitrotoluene-4-sulfonic acid) by continuous hydrogenation reduction of 6-chloro-3-nitrotoluene-4-sulfonicacid liquid phase
A technology of nitrotoluene and sulfonic acid liquid, applied in the preparation of sulfonic acid, organic chemistry, etc., can solve the problems of low reduction yield, large catalyst loss and loss, low product purity, etc., and achieves improved catalytic activity, improved conversion rate, The effect of suppressing the dechlorination reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
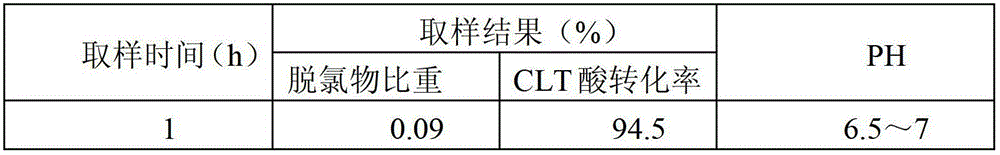
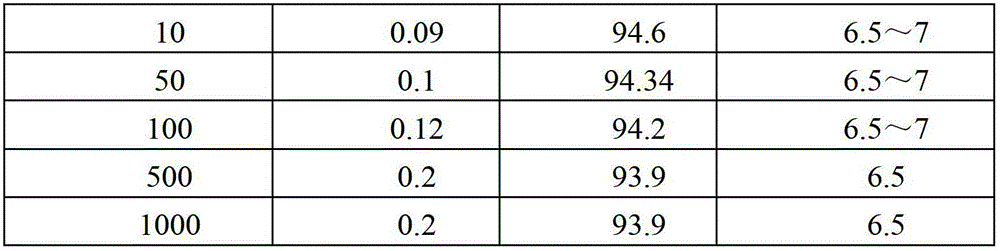
[0035] This embodiment adopts Pt / Al 2 o 3 Catalyst, the catalyst is based on Raschig ring type Al 2 o 3 As a carrier, the active component Pt is loaded on its surface, and the active component Pt is eggshell-shaped distribution on the surface of the carrier, and the mass of the active component Pt accounts for 0.2% of the mass of the carrier. The following is the use of the Pt / Al 2 o 3 Catalyst, the specific process of obtaining CLT acid by continuous hydrogenation reduction reaction in a fixed bed reactor.
[0036] (1) Add 50 ml of Pt / Al to the fixed bed reactor 2 o 3 catalyst, the Pt / Al 2o 3 Catalyst reduction at 300°C for 2 hours;
[0037] (2) The temperature of the system is raised to 80°C, hydrogen is continuously fed into the system, the pressure of the hydrogen is raised to 4MPa, and the saturated 6-chloro-3-nitrotoluene-4-sulfonic acid with a concentration of 5% by weight is continuously input by a metering pump Calcium solution, the input volume is set to 0....
Embodiment 2
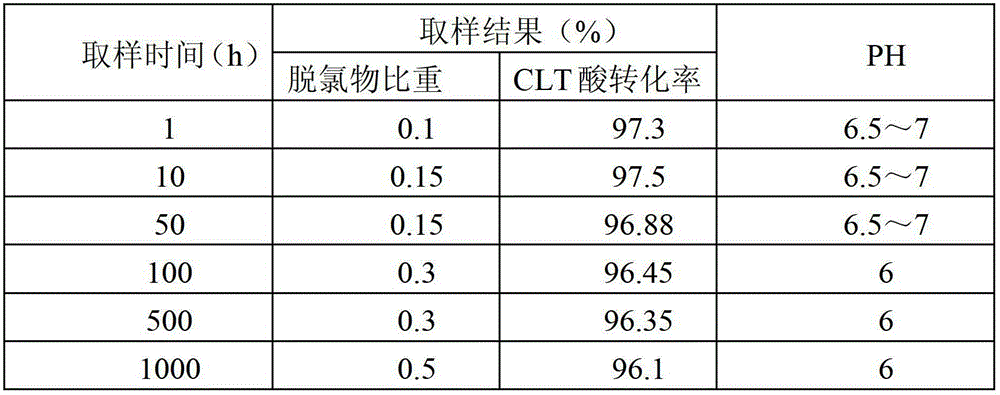
[0042] This embodiment adopts Pt / Al 2 o 3 Catalyst, the catalyst is based on Raschig ring type Al 2 o 3 As a carrier, the active component Pt is loaded on its surface, and the active component Pt is eggshell-shaped distribution on the surface of the carrier. The mass of the active component Pt accounts for 0.3% of the mass of the carrier, and the specific surface area of the carrier is 105 meters 2 / g, the pore volume is 0.6 ml / g carrier. The following is the use of the Pt / Al 2 o 3 Catalyst, the specific process of obtaining CLT acid by continuous hydrogenation reduction reaction in a fixed bed reactor.
[0043] (1) Add 50 ml of Pt / Al to the fixed bed reactor 2 o 3 catalyst, the Pt / Al 2 o 3 Catalyst reduction at 300°C for 2 hours;
[0044] (2) The temperature of the system is raised to 120°C, hydrogen gas is continuously fed into the system, and the pressure of the hydrogen gas rises to 4MPa, and a metering pump is used to continuously input saturated 6-chloro-3-ni...
Embodiment 3
[0048] This embodiment adopts Pt / Al 2 o 3 Catalyst, the catalyst is porous cylindrical Al 2 o 3 As a carrier, the active component Pt is loaded on its surface, and the active component Pt is eggshell-shaped distribution on the surface of the carrier. The mass of the active component Pt accounts for 0.4% of the mass of the carrier, and the specific surface area of the carrier is 106 m 2 / g, the pore volume is 0.6 ml / g carrier. The following is the use of the Pt / Al 2 o 3 Catalyst, the specific process of obtaining CLT acid by continuous hydrogenation reduction reaction in a fixed bed reactor.
[0049] (1) Add 100ml Pt / Al to the fixed bed reactor 2 o 3 catalyst, the Pt / Al 2 o 3 Catalyst reduction at 300°C for 2 hours;
[0050] (2) The temperature of the system is raised to 100°C, hydrogen is continuously fed into the system, the pressure of the hydrogen is raised to 4MPa, and the metering pump is used to continuously input saturated 6-chloro-3-nitrotoluene-4-sulfonic ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com