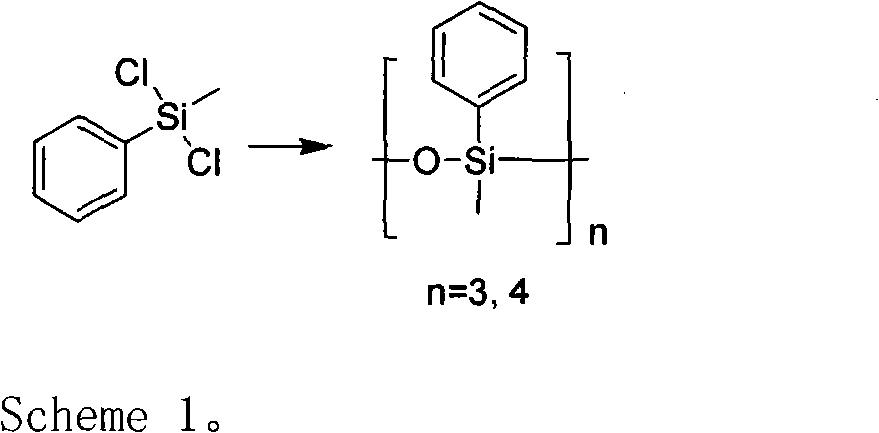
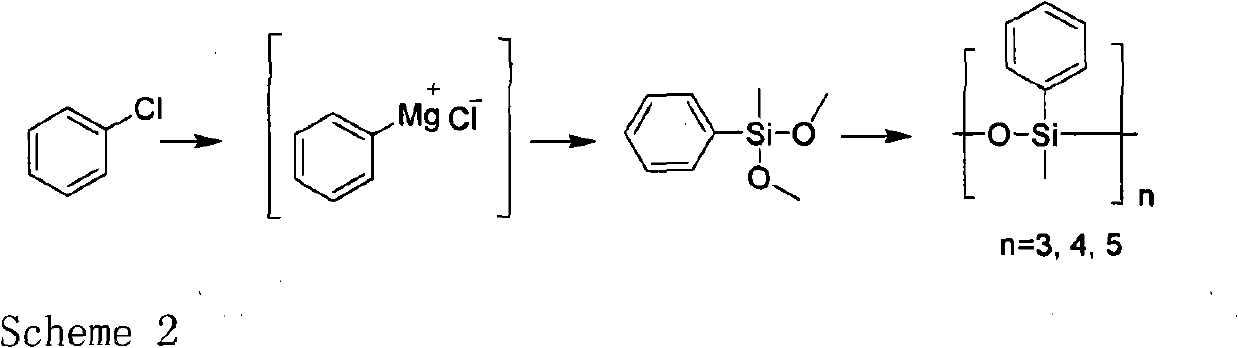
A preparation method for methyl phenyl siloxane unit-containing organosilicone ring bodies
A technology of methyl phenyl silicon and methyl phenyl dimethoxy, which is applied in the field of preparation of methyl phenyl silicon oxygen rings, can solve the problems of easy hydrolysis, excessive waste, unstable raw materials, etc., and achieve low cost , stable process and short reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The first reaction: preparation of intermediate methyl phenyl dimethoxy silane
[0021] Under nitrogen protection, add Mg (1.07kg, 44.64mol), 300ml of tetrahydrofuran, 4 grains of iodine, and heat to 70°C. After 40 minutes, slowly add chlorobenzene (5kg, 44.64mol) and The solution prepared by 14L tetrahydrofuran was added dropwise while keeping the reflux state, the internal temperature was kept at 65°C, and the dripping was completed for about 7 hours. After dripping, the solution was kept refluxed. After the reaction for 3 hours, it was naturally cooled to room temperature.
[0022] Under nitrogen protection, add methyltrimethoxysilane (18.24kg, 133.9mol) to a dry 50L reaction kettle, keep the temperature at about 10℃, and add phenylmagnesium chloride format reagent dropwise for about 6 hours and react at room temperature for 3 hours .
[0023] 15L of petroleum ether was added to the reaction kettle for extraction three times, the organic layers were combined, the dry solve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com