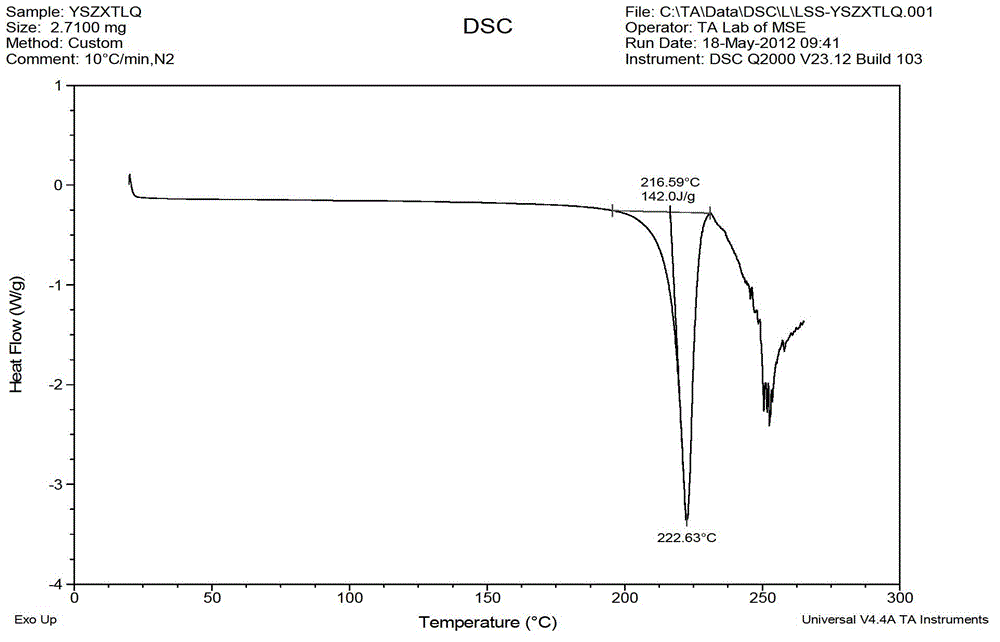
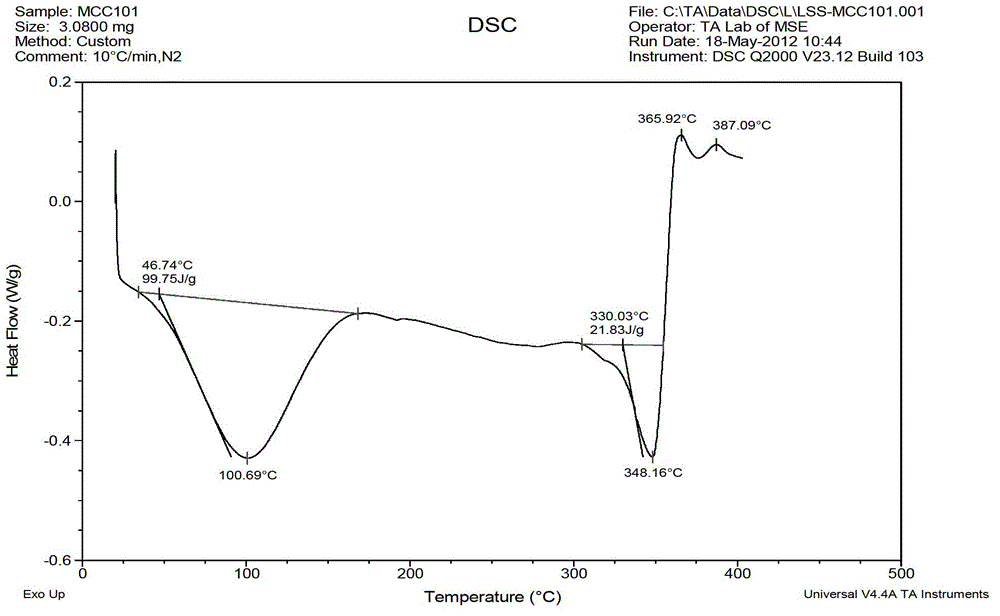
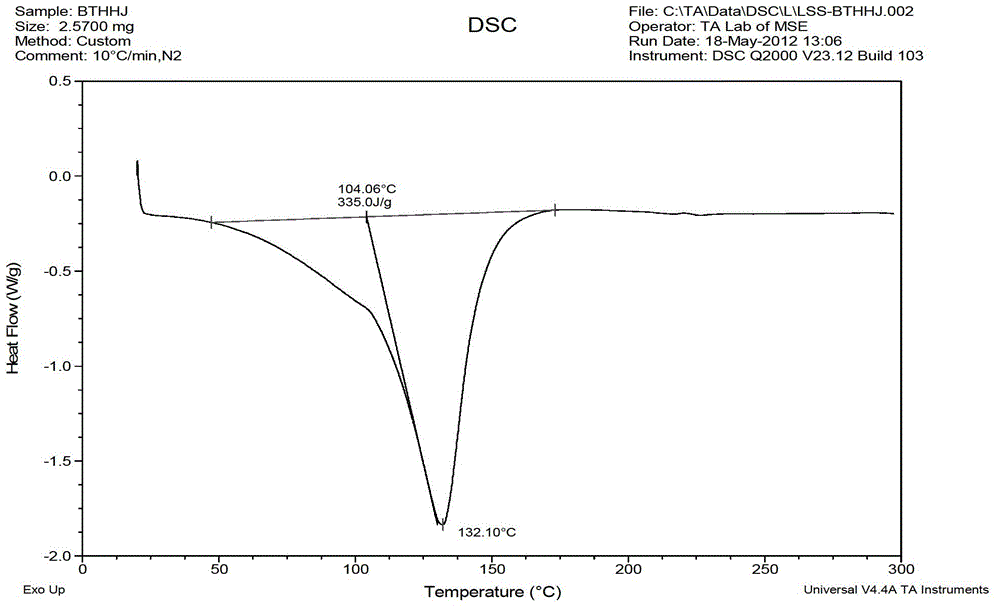
Levocetirizine hydrochloride chewable tablet and preparation method thereof
A technology of levocetirizine hydrochloride and chewable tablets, which can be applied to medical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as severe bitterness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment 1 (reference)
[0089] prescription:
[0090] Name of raw material
Prescription quantity (1000 tablets)
2.5g
β-cyclodextrin
5g
Microcrystalline Cellulose (Heidemer)
15g
water
Appropriate amount
100g
Directly compressed lactose
50g
Microcrystalline Cellulose PH102
50g
Crospovidone
10g
4g
Cantaloupe Flavor
5g
3g
[0091] Preparation Process:
[0092] 1. Weigh the prescribed amount of levocetirizine hydrochloride, put it in an appropriate amount of water, stir to dissolve, and set aside.
[0093] 2. Weigh the prescribed amount of β-cyclodextrin and microcrystalline cellulose (Haidemer) with an average particle size of 42 microns, mix well, add levocetirizine hydrochloride aqueous solution, stir well, and granulate with a 30-mesh sieve...
Embodiment 2
[0105] Embodiment 2 (the present invention)
[0106] prescription:
[0107] Drug-containing layer:
[0108]
[0109] Auxiliary layer:
[0110]
[0111] Preparation Process:
[0112] 1. Preparation of particles
[0113] Preparation of drug-containing layer:
[0114] 1. Weigh the prescribed amount of levocetirizine hydrochloride, put it in an appropriate amount of water, stir to dissolve, and set aside.
[0115] 2. Weigh the prescribed amount of β-cyclodextrin and microcrystalline cellulose, mix them evenly, add levocetirizine hydrochloride aqueous solution, stir evenly, granulate with a 30-mesh sieve, dry at 50°C, and sieve through a 30-mesh sieve grain.
[0116] 3. According to the amount of clathrate prepared, add direct compression lactose, microcrystalline cellulose PH102, and crospovidone according to the prescription amount, and mix well.
[0117] 4. Add the prescribed amount of magnesium stearate and mix evenly to obtain drug-containing layer granules.
[0...
Embodiment 3
[0131] Embodiment 3 (preferred embodiment of the present invention)
[0132] prescription:
[0133] Drug-containing layer:
[0134] Name of raw material
Prescription quantity (1000 tablets)
2.5g
β-cyclodextrin
15g
Microcrystalline Cellulose (Heidemer)
15g
water
Appropriate amount
Directly compressed lactose
150g
Microcrystalline Cellulose PH102
50g
Crospovidone
10g
3g
[0135] Auxiliary layer:
[0136] Name of raw material
Prescription quantity (1000 tablets)
150g
Microcrystalline Cellulose (Heidemer)
50g
lemon yellow
0.5g
water
Appropriate amount
4g
Cantaloupe Flavor
5g
Magnesium stearate
2g
[0137] Preparation Process:
[0138] 1. Preparation of particles
[0139] Preparat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com