Method for preparing aluminum hydroxide powder by esterification reaction
A technology of aluminum hydroxide and esterification, applied in the field of chemical production, can solve the problems of inability to achieve horizontal dispersion of molecules, difficult to achieve uniform dispersion of water, etc., and achieve strong promotion and application value, morphology rules, and post-processing processes. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
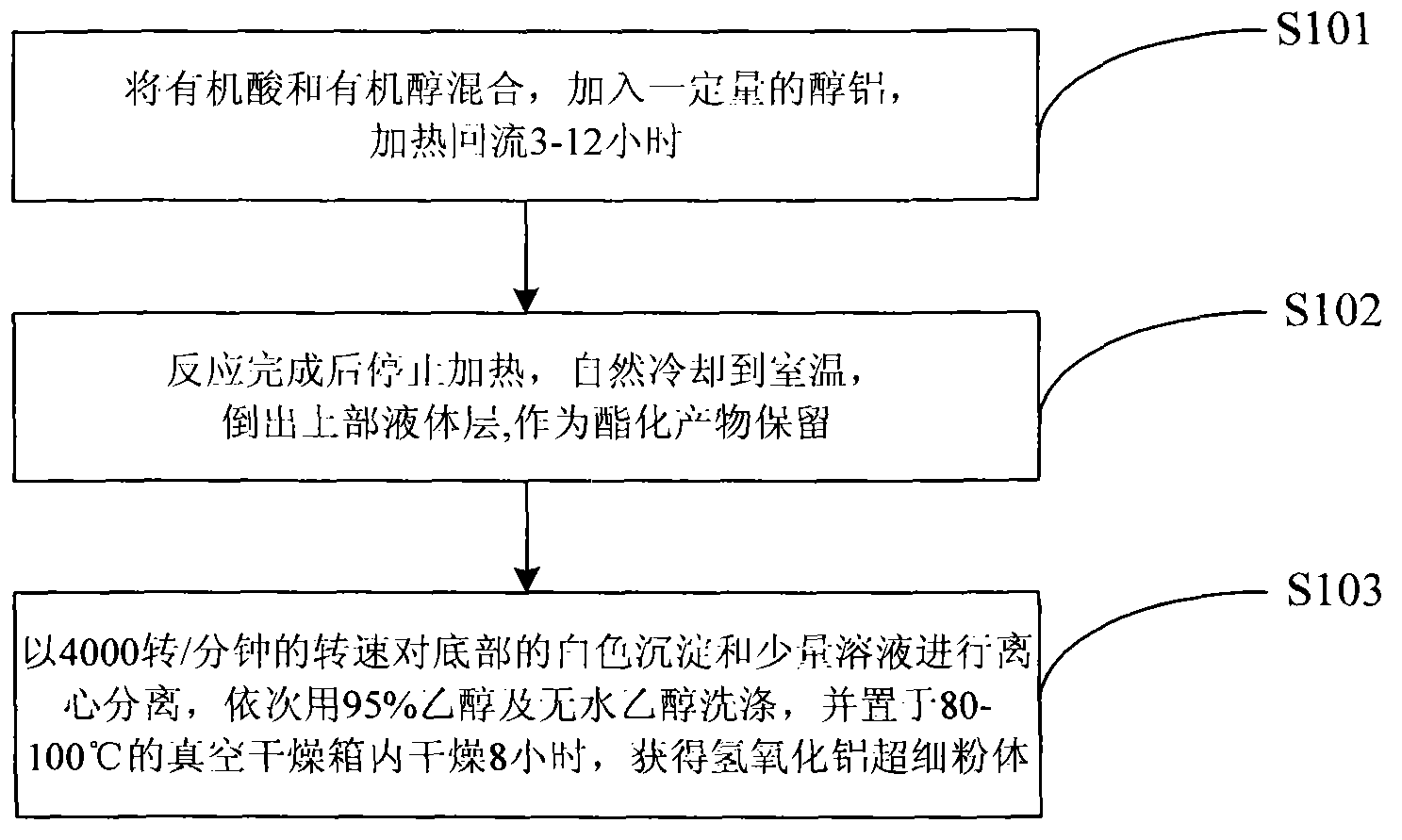
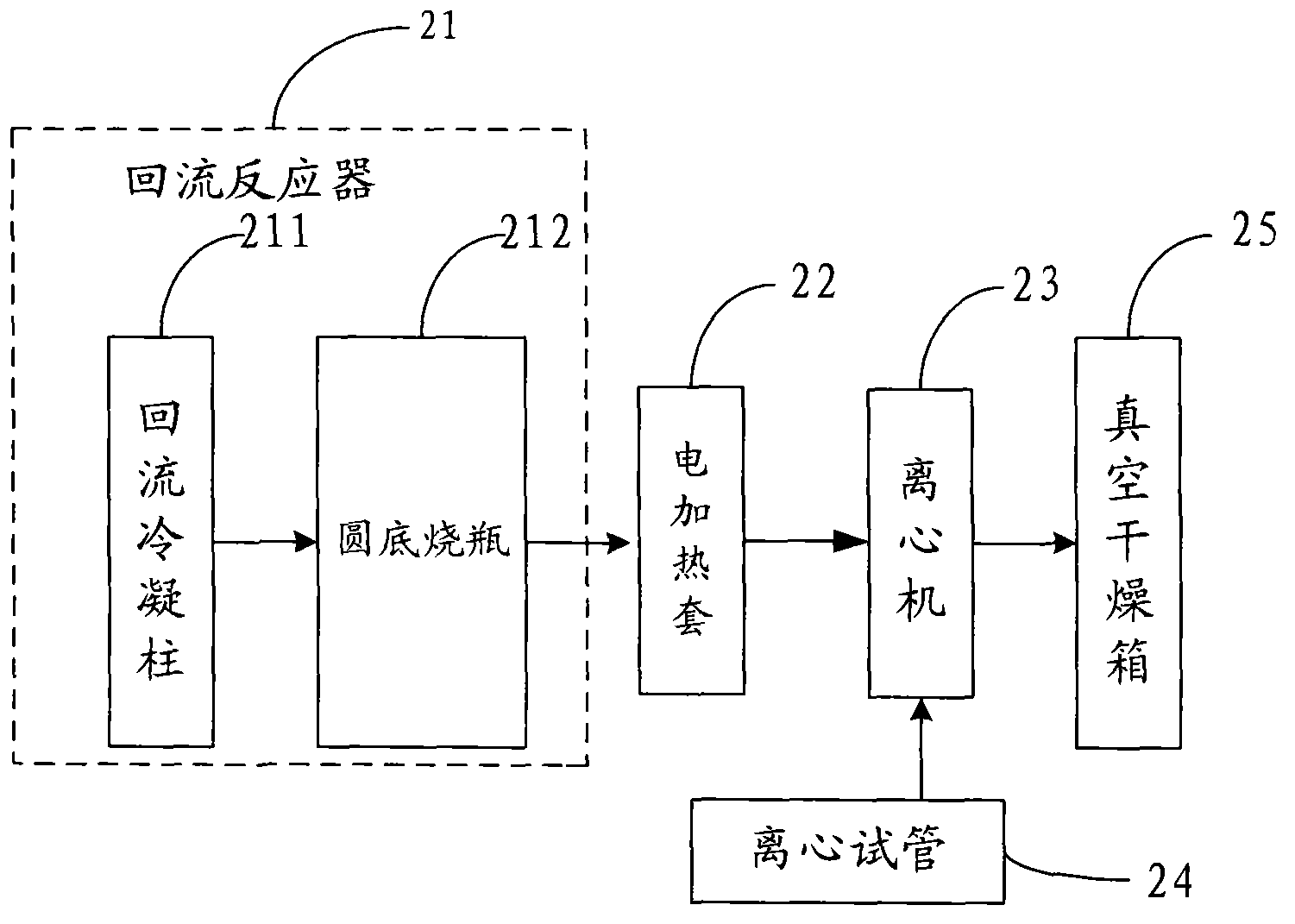
[0044]Embodiment 2: At first, 30ml (0.5mol) glacial acetic acid and 45ml isopropanol are joined in the round bottom flask 212, and the theoretical water yield of the esterification reaction of this embodiment is 0.5mol, namely 9 grams, considering the hydrolysis of aluminum alkoxide required theoretical water consumption, and properly leave a margin, so add 0.1 mole of aluminum isopropoxide, the degree of filling in the round bottom flask 212 is controlled at 75%; install the reflux condensation column 211, place the reflux reactor 21 in Heat and reflux in the electric heating mantle 22 for 8 hours; secondly, after the reaction is completed, stop heating, cool to room temperature naturally, pour out the liquid layer on the top of the round bottom flask 212, and keep it as an esterification product; then, remove the white precipitate at the bottom and a small amount of The solution is poured into a centrifuge test tube 24, put into a centrifuge 23, and centrifuged at a speed of ...
Embodiment 3
[0045] Embodiment 3: First, 30ml (0.5mol) glacial acetic acid and 50ml isobutanol are added in the round bottom flask 212, and the theoretical water yield of the esterification reaction of the present embodiment is 0.5mol, namely 9 grams, considering the hydrolysis of aluminum alkoxide required theoretical water consumption, and properly leave a margin, so add 0.1 mole of aluminum isobutoxide, the degree of filling in the round bottom flask 212 is controlled at 80%; install the reflux condensation column 211, place the reflux reactor 21 in Heat and reflux in the electric heating mantle 22 for 6 hours; secondly, after the reaction is completed, stop heating, cool to room temperature naturally, pour out the liquid layer on the top of the round bottom flask 212, and keep it as an esterification product; then, remove the white precipitate at the bottom and a small amount of The solution is poured into a centrifuge test tube 24, put into a centrifuge 23, and centrifuged at a speed o...
Embodiment 4
[0046] Embodiment 4: First, 45ml (0.6mol) of propionic acid and 35ml of absolute ethanol are added in the round bottom flask 212, and the theoretical water yield of the esterification reaction in this embodiment is 0.6mol, i.e. 10.8 grams, taking into account the hydrolysis of aluminum alkoxide required theoretical water consumption, and properly leave a surplus, so add 0.1 mole of aluminum triethoxide, the degree of filling in the round bottom flask 212 is controlled at 80%; install the reflux condensation column 211, place the reflux reactor 21 in the Heat and reflux in the heating mantle 22 for 6 hours; secondly, after the reaction is completed, stop heating, cool to room temperature naturally, pour out the liquid layer on the top of the round bottom flask 212, and keep it as an esterification product; then, remove the white precipitate at the bottom and a small amount of solution , poured into the centrifuge test tube 24, put into the centrifuge 23, centrifuged at a speed o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com