Alkylene bialkylphenol compound and preparation method thereof
A technology for alkyldialkylphenols and compounds, which is applied in the field of phenolic compounds and their preparation, can solve the problems of complex operation, high production cost, low yield and purity, and achieve high product purity, low cost and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
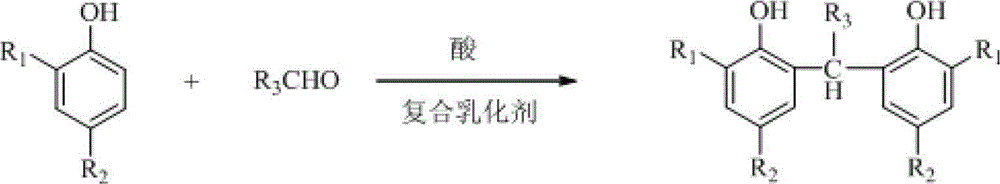
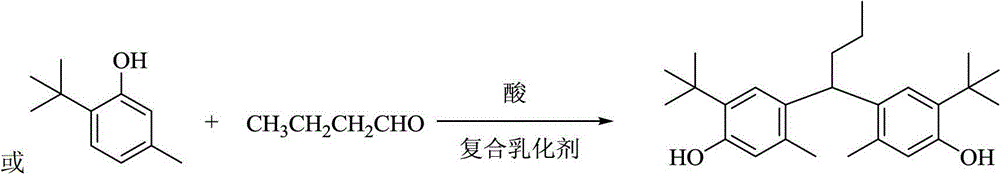
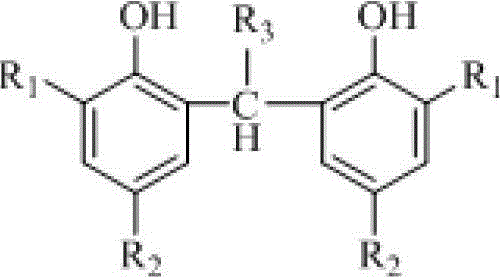
[0023] In a 2L four-neck flask equipped with a stirring thermometer and a reflux condenser, under nitrogen protection, add 480g of 2% sulfuric acid aqueous solution (200g / 100g phenol), 6g of polyvinyl alcohol (2.5g / 100g phenol), and stir to raise the temperature to 45°C. Add 4.8g of sodium dodecylsulfonate (2.0g / 100g of phenol), 240g of 2,4-di-t-amylphenol, 90g of 40% acetaldehyde (the molar ratio of phenol to fatty aldehyde is 1:0.8), and then raise the temperature To 90 ~ 95 ℃, heat preservation reaction 5h. Cool down to room temperature and filter. The filter cake was washed with water to become neutral, and dried at room temperature to obtain 2,2'-ethylenebis(4,6-di-t-pentylphenol), calculated on the basis of 2,4-di-t-pentylphenol, with a yield of 98.0 %, purity 98.5%.
Embodiment 2
[0025] In a 2L four-necked flask equipped with a stirring thermometer and a reflux condenser, under the protection of nitrogen, add 600g of 2% sulfuric acid aqueous solution (333g / 100g phenol), 3g of polyvinyl alcohol (1.7g / 100g phenol), and stir to raise the temperature to 45°C. Add 1.8g of sodium lauryl sulfate (1.0g / 100g phenol), 180g of 2,4-di-t-amylphenol, 50g of 40% acetaldehyde (the molar ratio of phenol to fatty aldehyde is 1:0.59), and then heat up to 95~100℃, heat preservation reaction for 9h. Cool down to room temperature and filter. The filter cake was washed with water to become neutral, and dried at room temperature to obtain 2,2'-ethylenebis(4,6-di-t-pentylphenol), calculated on the basis of 2,4-di-t-pentylphenol, with a yield of 98.0 %, purity 98.7%.
Embodiment 3
[0027] In a 2L four-necked flask equipped with a stirring thermometer and a reflux condenser, under nitrogen protection, add 500g of 5% sulfuric acid aqueous solution (278g / 100g phenol), 3g of polyvinyl alcohol (1.7g / 100g phenol), and stir to raise the temperature to 45°C. Add 2.7g sodium dodecylbenzenesulfonate (1.5g / 100g phenol), 180g 2,4-di-tert-butylphenol, 45g 40% formaldehyde (the molar ratio of phenol to fatty aldehyde is 1:0.69), then raise the temperature To 80 ~ 90 ℃, heat preservation reaction 8h. Cool down to room temperature and filter. The filter cake was washed with water to become neutral, and dried at room temperature to obtain 2,2'-methylenebis(4,6-di-tert-butylphenol). Based on 2,4-di-tert-butylphenol, the yield was 98.2 %, purity 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com