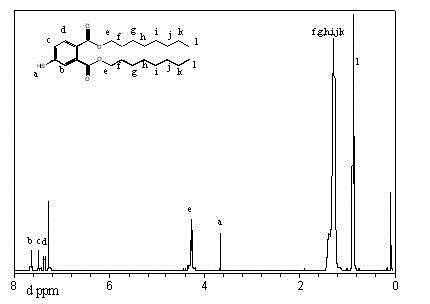
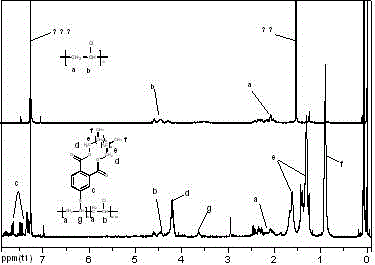
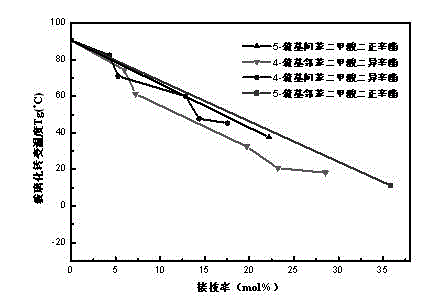
Polyvinyl chloride plasticizer and preparation method thereof
A polyvinyl chloride and plasticizer technology, applied in the field of polyvinyl chloride plasticizer and its preparation, can solve problems such as health threats, pollution of physiological fluids, loss of initial properties of PVC, etc., to expand the application range and reduce the glass transition The effect of temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Synthesis of 4-chlorosulfonylphthaloyl chloride
[0038] Add 100 g of sodium phthalate-4-sulfonate into a 1L round bottom flask, and add 220 g of PCl under stirring 5 , heated in an oil bath at 180°C, and reacted for 15 hours; then, 500ml of toluene solution was added to the reactor, heating was stopped, and stirring was continued. After standing for a period of time, white NaCl precipitated out, filtered, and distilled under reduced pressure to obtain 4-chlorosulfonylphthaloyl chloride.
[0039] (2) Synthesis of 4-chlorosulfonyl phthalate di-isooctyl
[0040] Take 33.2mmol of the product from the previous step, put it in a 500ml round bottom flask, add 250ml of chloroform and stir to dissolve, add 66.4mmol of 2-ethylhexanol and 66.4mmol of triethylamine successively under stirring, and react at 65°C for 2 hours. The product organic phase was washed with 1M HCl solution, saturated NH 4 Cl solution and washed with deionized water. Then the organic phases were com...
Embodiment 2
[0044] (1) Synthesis of 5-chlorosulfonylisophthaloyl chloride
[0045]Add 80 g of isophthalic acid-5-sodium sulfonate into a 1L round bottom flask, and add 240 g of PCl under stirring 5 , and reacted for 20 hours at the temperature of an oil bath at 170° C.; then, 500 ml of toluene solution was added to the reactor, heating was stopped, and stirring was continued. After standing for a period of time, white NaCl precipitated out, filtered, and distilled under reduced pressure to obtain 5-chlorosulfonylisophthaloyl chloride.
[0046] (2) Synthesis of 5-chlorosulfonyl diisooctyl isophthalate
[0047] Take 33.2mmol of the product 5-chlorosulfonyl-isophthaloyl chloride from the previous step, put it in a 500ml round-bottomed flask, add 250ml of chloroform and stir to dissolve, then add 99.6mmol of 2-ethylhexanol and 99.6mmol of trichloromethane under stirring Ethylamine, reacted at 55°C for 4 hours. The organic phase of the product was sequentially washed with 1mol / l HCl solutio...
Embodiment 3
[0051] (1) Synthesis of 4-chlorosulfonylphthaloyl chloride
[0052] This step is the same as step (1) of Example 1.
[0053] (2) Synthesis of 4-chlorosulfonyl phthalate di-n-octyl
[0054] Take 33.2mmol of the product from the previous step, put it in a 500ml round bottom flask, add 250ml of chloroform and stir to dissolve, add 66.4mmol of n-octanol and 66.4mmol of pyridine successively under stirring, and react at 70°C for 1 hour. The product organic phase was washed with 1M HCl solution, saturated NH 4 Cl solution and washed with deionized water. Then the organic phases were combined, dried over anhydrous magnesium sulfate, filtered, and the crude product obtained by concentration was distilled under reduced pressure to obtain 4-chlorosulfonyl phthalate di-n-octyl ester.
[0055] (3) Synthesis of 4-mercaptophthalate di-n-octyl
[0056] Take 10.2mmol of the product from the previous step and place it in a 250ml round bottom flask, dissolve it with 5.0ml acetic acid, and d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com