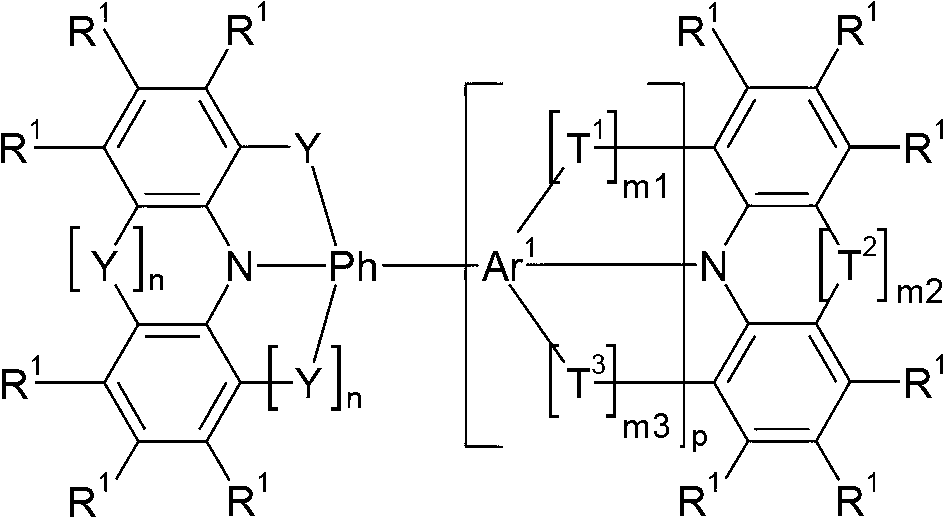
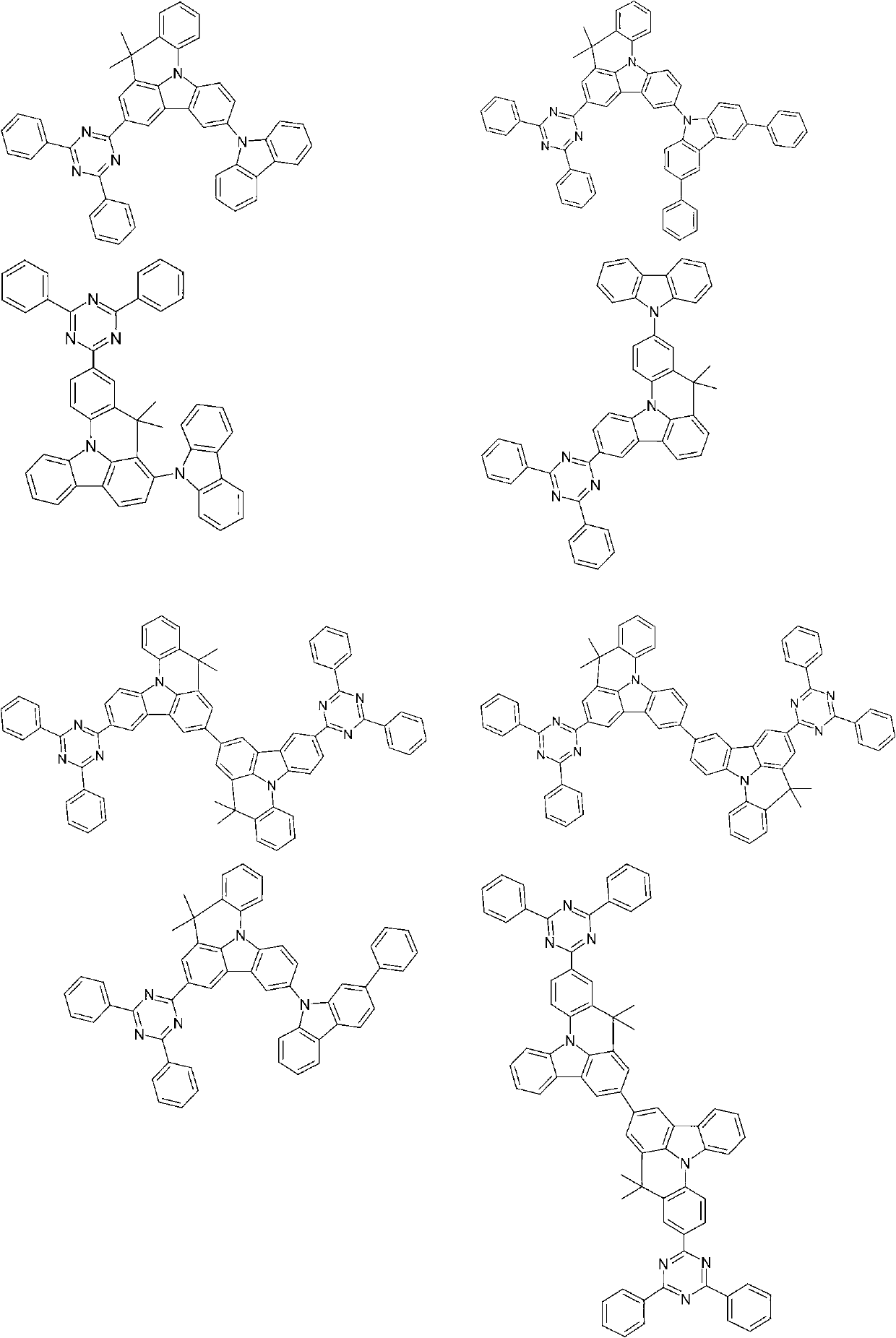
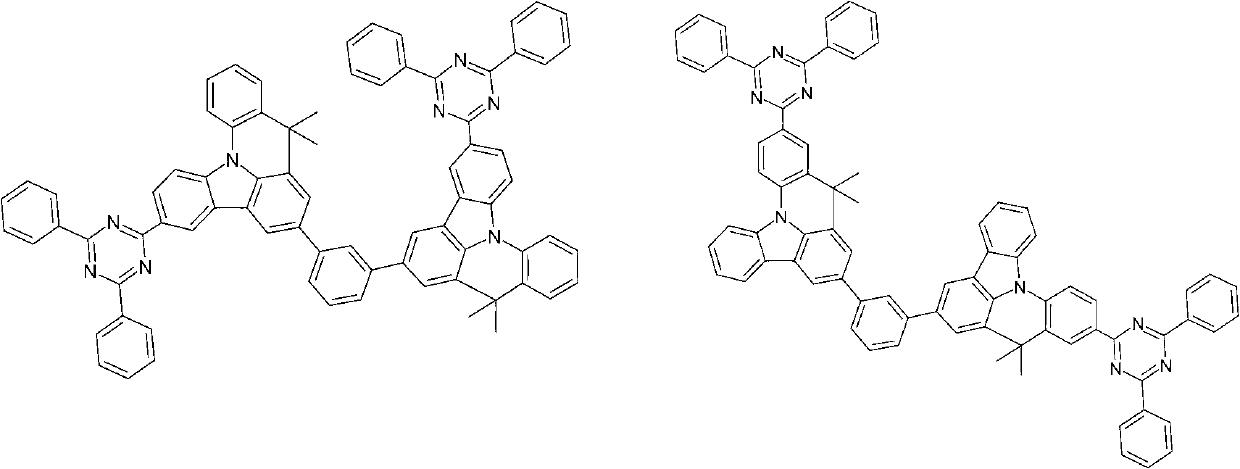
Compounds for electronic devices
A compound and atomic technology, applied in the field of electronic devices, can solve problems such as high technical complexity, industrial process complexity, thermal instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 30
[0306] A) Synthesis of the compounds of the present invention from Examples 1 to 30
[0307] Unless otherwise noted, the following syntheses were carried out under a protective gas atmosphere. This starting material can be purchased from ALDRICH or ABCR (palladium(II) acetate, tri-o-tolylphosphine, inorganics, solvents). According to the literature, 8,8-dimethylindolo[3,2,1-de]acridine and 7,7,11,11-tetramethyl-7H,11H-benzo[1,8]indole can be synthesized Indo[2,3,4,5,6-de]acridine (Chemische Berichte 1980, 113(1), 358-84). 8H-indolo[3,2,1-de]phenazine (Journal of the Chemical Society 1958, 4492-4) and B-[4-(1-phenyl-1H-benzimidazol-2-yl) phenyl]boronic acid (Advanced Functional Materials 2008, 18(4), 584-590), 2-bromoindolo[3,2,1-jk]carbazole and indolo[3,2,1-jk] Carbazoleboronic acid (Chemistry A European Journal, 2009, 15(22), 5482-5490), N-[1,1'-biphenyl]-4-yl-9,9-dimethyl-9H-fluorene-2 -amine (WO 2006073054) and 7-bromo-2,12-dimethyl-10-phenyl-10,12-dihydro-10-azaideno[...
Embodiment 1
[0308] Example 1: 3-bromo-8,8-dimethyl-8H-indolo[3,2,1-de]acridine
[0309]
[0310] Methyl 2-(3-bromo-9H-carbazole)benzoate:
[0311]In 2000ml of DMF, 62g (207mmol) of methyl 2-(9H-carbazole)benzoate was cooled to -10°C, 37.3g (207mmol) of NBS was added in portions, and the mixture was stirred at room temperature for 6h. Subsequently, 500 ml of water was added to the mixture, followed by CH 2 Cl 2 extract. The organic phase was dried using magnesium sulfate and the solvent was removed under vacuum. The product is washed with hot toluene by stirring and filtered with suction.
[0312] Yield: 72 g (190 mmol), 92% of theory, according to 1 The purity by H-NMR is about 98%.
[0313] 2-[2-(3-Bromocarbazol-9-yl)phenyl]propan-2-ol:
[0314] 81 g (213 mmol) of methyl 2-(3-bromo-9H-carbazole)benzoate were dissolved in 1500 ml of dry tetrahydrofuran and degassed. The mixture was cooled to -78°C and 569 ml (854 mmol) of methyllithium were added over a period of 40 minutes. T...
Embodiment 2
[0318] Example 2: 6-bromo-8,8-dimethyl-3-phenyl-8H-indolo[3,2,1-de]acridine
[0319]
[0320] Methyl 2-(3-phenyl-9H-carbazole)benzoate:
[0321] 85g (350mmol) 3-phenyl-9H-carbazole, 63ml (262mmol) methyl 2-iodobenzoate, 87g (631mmol) potassium carbonate and 9.3g (35mmol) 18-crown-6 were first under protective gas Introduce into 1200ml of DMF and heat at 130°C for 86h. The mixture was then evaporated, washed with hot heptane by stirring, chromatographed (heptane / CH 2 Cl 2 1:1) for purification. The product was washed with hot hexane with stirring and filtered with suction.
[0322] Yield: 82 g (219 mmol), 62% of theory, according to 1 The purity by H-NMR is about 97%.
[0323] Methyl 2-(3-bromo-6-phenyl-9H-carbazole)benzoate:
[0324] Cool 78.4g (207mmol) of methyl 2-(3-phenyl-9H-carbazole)benzoate in 2000ml of DMF to -10°C, add 37.3g (207mmol) of NBS in portions, and stir the mixture at room temperature 6h. Subsequently, 500 ml of water was added to the mixture, fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com