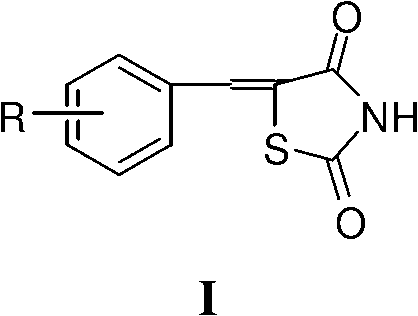
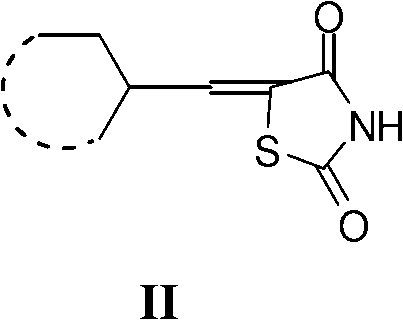
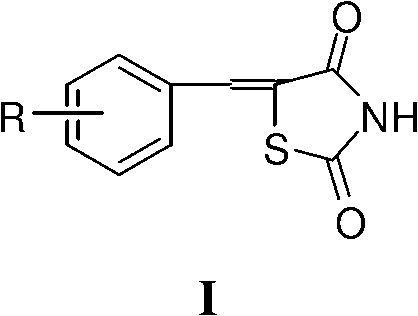
Application of 5- aryl (heterocycle) methylenethiazolidine-2,4-dione in preparation of PPAR (Peroxisome Proliferator Activated Receptor) agonist
A technology of heterocyclic methylene thiazolidine and aryl methylene thiazolidine, which is applied in the field of chemical pharmaceuticals and can solve problems such as compound termination research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1, thiazolidine-2, the synthesis of 4-dione
[0019]
[0020] Add chloroacetic acid, thiourea and concentrated hydrochloric acid (the amount ratio of the three is 1mol: 1.1mol: 250ml) into the reaction flask, heat and reflux for 3 hours, cool and stir to crystallize, filter, and wash the crystals with water to obtain 2-imino-4 -thiazolone hydrochloride; then add an appropriate amount of water and a certain amount of activated carbon to the crystal, heat and reflux for 2 m hours, filter while it is hot, cool and stir the filtrate to crystallize, filter, wash the crystal with water, and dry to obtain thiazolidine-2, 4-diketone. The results of the synthesis experiments are shown in Table 1.
[0021] Table 1 Thiazolidine-2,4-dione synthetic experimental results
[0022]
[0023] Thiazolidine-2,4-dione: white crystals; IR (KBr, cm -1 ): 3134(s, v NH ), 3047(s, v =CH ), 2949, 2825 (s, v CH2 ), 1739 (s, v C=O ), 1655 (s, v C=O ), 618.0 (m, v C-S ); 1...
Embodiment 2
[0025] Embodiment 2, the synthetic (solid phase condensation method) of target compound TM-7
[0026]
[0027] Add aldehyde, thiazolidine-2,4-dione and anhydrous sodium acetate (the molar ratio of the three is 1.1:1.0:1.0) into the mortar, mix and grind to powder, then transfer to the reaction bottle, oil bath 125 ℃ reaction, it can be seen that the solid raw material melts rapidly, and then thickens. Stop the reaction when the solidification is complete. 6. After stirring at room temperature for 30 minutes, let stand at 4°C, filter with suction, wash the filter cake with water, dry at 100°C, disperse with ether-ethyl acetate (volume ratio 2:1) mixed solvent overnight, filter with suction, and dry to obtain Target compound TM-7. The results of the synthesis experiments are shown in Table 2.
[0028] Table 2 The synthesis experiment result of target compound TM-7
[0029]
[0030]
[0031] After retrieval, the TM-7 series of target compounds are all known compounds ...
Embodiment 3
[0070] Embodiment 3, the synthesis of target compound TM-8
[0071] 1. Solid phase condensation method
[0072]
[0073] Add aldehyde, thiazolidine-2,4-dione and anhydrous sodium acetate (the molar ratio of the three is 1.1:1.0:1.0) into the mortar, mix and grind to powder, then transfer to the reaction bottle, oil bath 125 ℃ reaction, it can be seen that the solid raw material melts rapidly and then thickens, stop the reaction when the solidification is complete, add an appropriate amount of DMF while it is hot to completely dissolve the solid, add water to precipitate a large amount of solid, adjust the pH to 5-6 with 2N HCl, and stir at room temperature for 30 minutes Stand at 4°C, filter with suction, wash the filter cake with water, dry at 100°C, disperse with ether-ethyl acetate (volume ratio 2:1) mixed solvent overnight, filter with suction, and dry to obtain the target compound TM-8. The synthesis experiment results are shown in Table 3.
[0074] 2. Liquid phase c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com