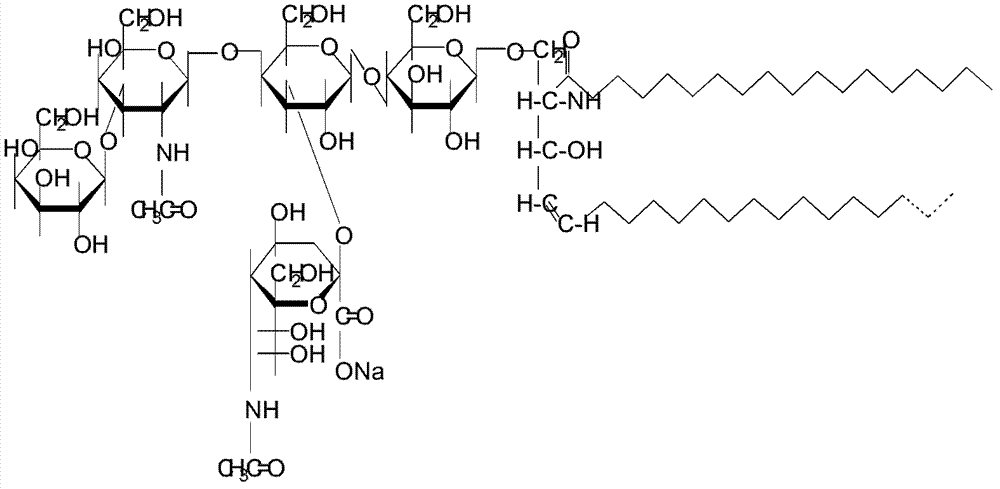
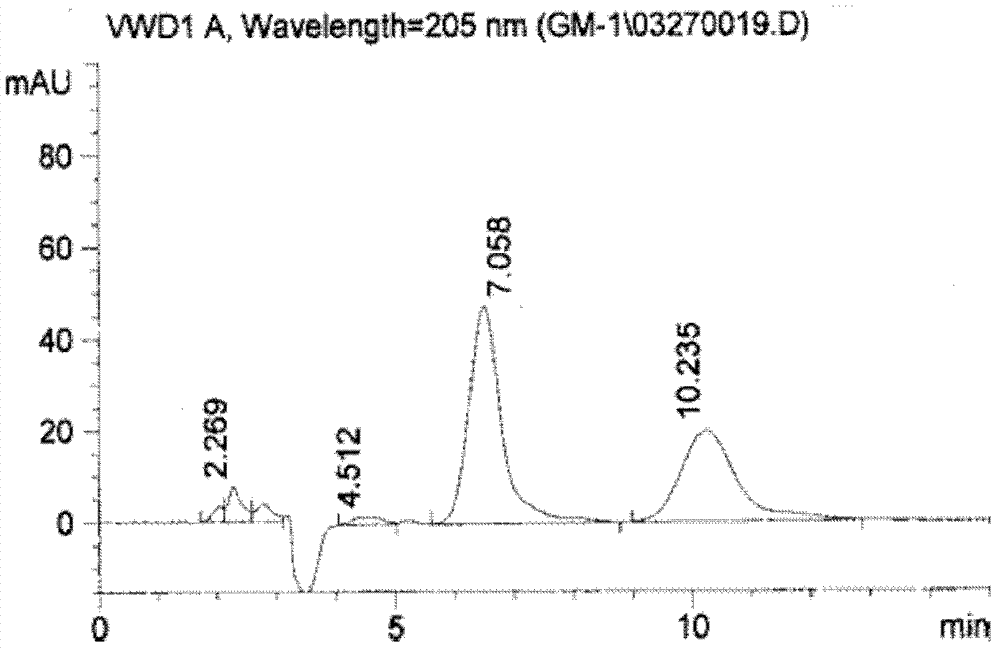
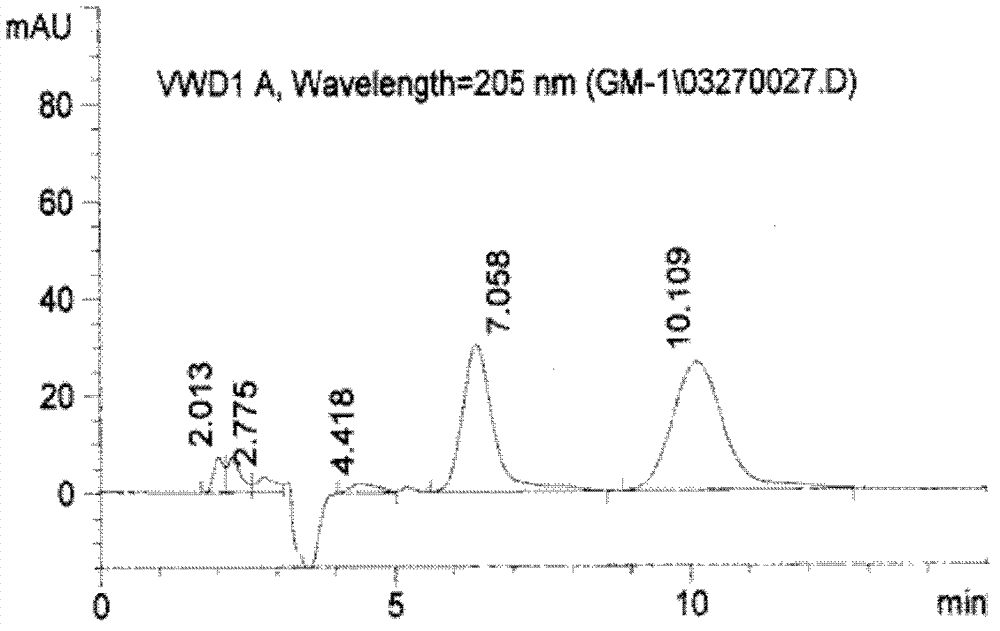
Preparation method of monosialoteterahexosylganglioside
A technology of ganglioside and monosialic acid, which is applied in the field of medicine and biology, can solve the problems of low yield and purity of GM1, large amount of organic solvent, and difficult waste disposal, etc., and achieves the reduction of water-soluble impurities, high recovery rate, and reduction of The effect of residual impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 pig brain selection and pretreatment
[0043] Select fresh pig brains or frozen pig brains that have passed the animal epidemic prevention inspection, remove meninges and blood vessels, wash away blood stains with deionized water, rinse 3 times, mince, and homogenate. After pulverization, use acetone equivalent to 5 times the volume of the pig brain used in the test, stir for 15 minutes, and stand at room temperature for immersion treatment for 12 hours. Filter and collect the filter residue. The filter residue was moved to a distillation pot for distillation and drying, and the dry residue was collected to obtain porcine brain acetone powder, which was sealed and subpackaged, and stored for later use.
Embodiment 2
[0044] Example 2 Extraction of total gangliosides
[0045] Pump 500L of extraction buffer into the stirring tank, then add 100 kg of pig brain acetone powder, stir and extract for 60 minutes, filter, and collect the precipitate and filtrate. The extraction buffer consists of chloroform, methanol and water in a volume ratio of 4:8: 3 ratio mix. Add 500L of the extraction buffer to the precipitate, stir and extract for 60 minutes, filter, and collect the precipitate and extract. Combine the filtrate and the extract, add water equivalent to 20% of the volume of the extract to the extract, and mix well to obtain a mixed solution; leave the mixed solution at room temperature for 12 hours to separate layers, and take the supernatant. Add NaOH powder to the supernatant so that the mass fraction of NaOH in the supernatant is 0.3%, stir and dissolve, the pH value reaches 10.0-11.0, saponify at 60° C. for 45 minutes, and cool the saponified solution for later use.
[0046] After the p...
Embodiment 3
[0047] The extraction of embodiment 3 GM1 crude product
[0048] Add 0.2% hydrochloric acid (that is, 120 ml) to 60 L of the concentrated ganglioside prepared in Example 2 to adjust the pH to 2.5 to obtain a mixed solution. Stir the reaction at 80°C for 2 hours, cool to room temperature, and set aside. Add acetone equivalent to 5 times the volume of the mixed solution, and cryoprecipitate at -10°C for 12 hours. Centrifuge, collect the precipitate by filtration with a 200-mesh filter cloth, and obtain a crude product containing GM1, which contains a mass fraction of 35% "crude product 1".
[0049] Purify "crude product 1": take 300 kilograms of crude silica gel, and use mobile phase chloroform / methanol / water (the volume ratio of chloroform, methanol and water is 45:40:5) to wet-pack to a 80×100cm chromatographic column middle. Fully dissolve "crude product 1" with 80L of mobile phase per 10kg, and load the sample at a flow rate of 50L / hour. Elute with the above-mentioned mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com