Cycloolefin copolymer and preparation method thereof
A cyclic olefin copolymer and cyclic olefin technology, applied in the polymer field, can solve the problems of severe brittleness, low catalytic activity, unfavorable application, etc., and achieve the effect of high transparency and good molecular weight distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention provides a kind of preparation method of cycloolefin copolymer described in above-mentioned technical scheme, comprises the following steps:
[0043] In an inert solvent, the cycloolefin monomer having the structure of formula (II) is polymerized with α-olefin in the presence of a catalyst to obtain a cycloolefin copolymer having the structure of formula (I);
[0044] Formula (II); Formula (I);
[0045] In formula (I), m and n are degrees of polymerization, m:n≥1.5;
[0046] R 1 and R 2 independently selected from hydrogen or saturated aliphatic hydrocarbon groups with 1 to 10 carbon atoms.
[0047] The preparation method of the cyclic olefin copolymer provided by the present invention is carried out in an inert solvent. The inert solvent is preferably a linear hydrocarbon compound, a cyclic hydrocarbon compound or an aromatic compound, more preferably a benzene compound, and most preferably toluene.
[0048] In the inert solvent, in the pr...
Embodiment 1
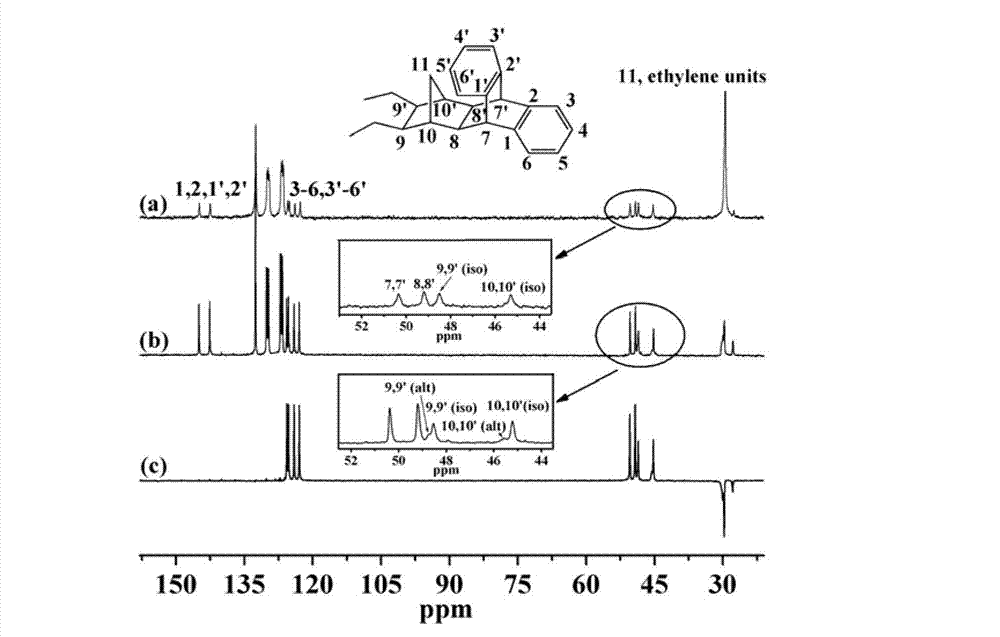
[0068] 42 g of anthracene and 108 g of norbornadiene were sequentially added into a 150 mL autoclave. Under nitrogen atmosphere, anthracene and norbornanol were reacted by heating at 180°C for 27 hours. After the reaction was completed, the temperature of the reaction system was lowered to room temperature, and the unreacted norbornadiene was evaporated to obtain a light yellow solid. Using petroleum ether as a solvent, the obtained light yellow solid was extracted with a Soxhlet extractor for 24 hours to obtain a concentrated extract. The concentrated extract was recrystallized to precipitate white crystals, and then filtered, and the resulting solid was vacuum-dried at 50° C. to obtain a cycloolefin monomer.
[0069] The cycloolefin monomer obtained in the present invention is a white crystal, the mass of the cycloolefin monomer obtained by weighing is 50.2 g, and the calculated yield is 80.0%.
[0070] The present invention conducts structural characterization of the obta...
Embodiment 2
[0072] Under an ethylene atmosphere, 21.5 mL of anhydrous toluene, 1 mL of a toluene solution with a structure of formula (II) prepared in Example 1 with a molar concentration of 2 mol / L, 1.5 mL of anhydrous toluene, and 1.5 mL of molar A toluene solution of TIBA with a concentration of 0.5mol / L and 3mL of [Ph 3 CB(C 6 f 5 ) 4 ], the resulting mixed solution was stirred at 40° C. for 10 minutes, then 3 mL of a toluene solution of CGC with a molar concentration of 1 μmol / mL was added thereto, and ethylene was continuously fed into the reactor, and the ethylene concentration was maintained. The pressure was 1 atmosphere, and the polymerization was carried out for 5 minutes. After the polymerization reaction is completed, the obtained reaction solution is poured into an ethanol solution of hydrochloric acid with a volume fraction of 10%, and the reaction product is precipitated; the obtained reaction product is filtered and washed 3 times with acetone, and the obtained product...
PUM
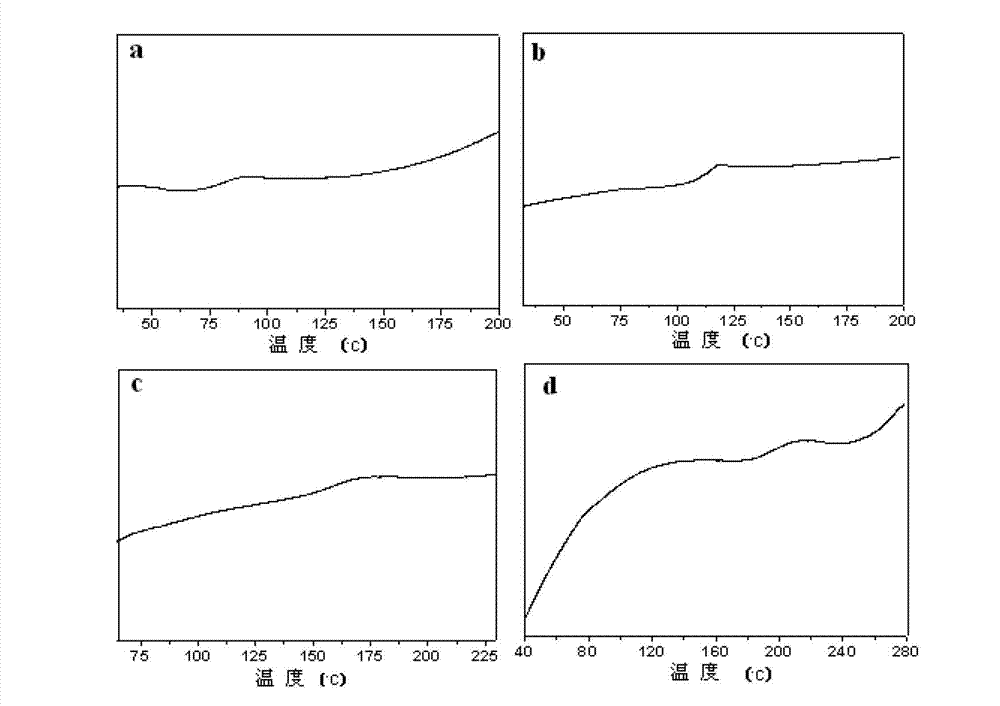
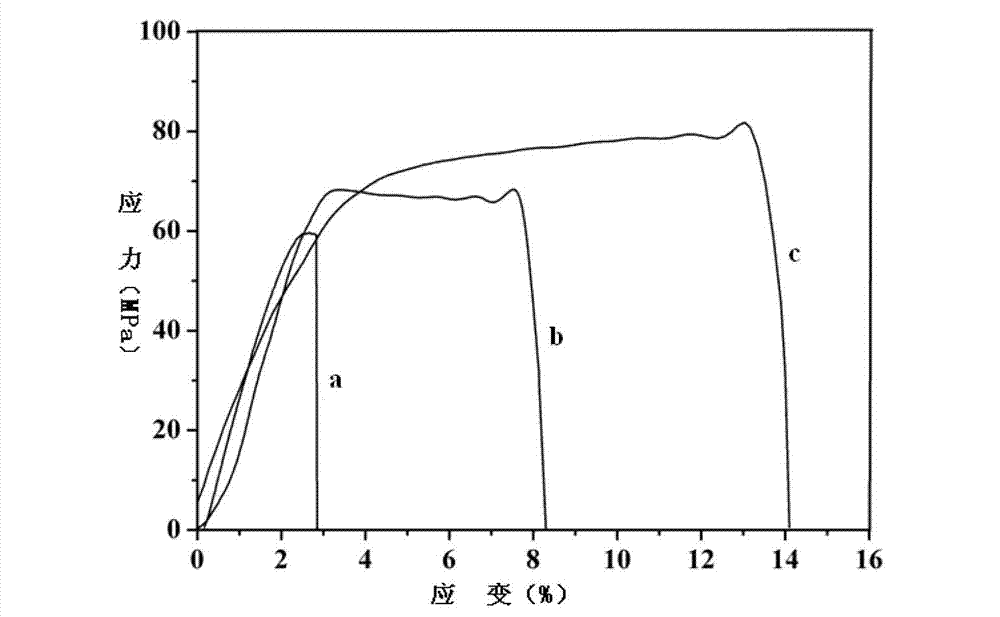
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com