Compound immunoenhancement agent, vaccine for birds and method for preparing compound immunoenhancement agent
An immune enhancer and compound technology, applied in the field of biomedicine, can solve the problems of inability to stimulate cellular immunity, short antibody maintenance time, low antibody titer, etc., to prolong the antibody duration, long antibody duration, and shorten the immune window period Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 compound immunopotentiator, poultry vaccine
[0032] (1) Test material
[0033] Polymyocytes, imiquimod, resimod, muramyl dipeptide, and antiparasitic drug levamisole were purchased from SIGMA. White oil was purchased from Esso, France, and Tween-80 and Span-80 were purchased from Guangdong Zhaoqing Chaoneng Industrial Co., Ltd. Inorganic salts for preparing phosphate buffer were purchased from Sinopharm Chemical Reagent Co., Ltd.
[0034] (2) Test method
[0035] Preparation of compound immune enhancer:
[0036] Preparation of aqueous phase solution: first prepare phosphate (PBS) buffer solution, its formula contains Na 2 HPO 4 1.44g / L, KH 2 PO 4 1.44g / L for NaCl, 8g / L for NaCl, and 0.2g / L for KCl. Dissolve the above salts in double distilled water, adjust the pH to 7.0, and sterilize for later use. Then in the PBS buffer solution, add polyinosinocytes, muramyl dipeptide, levamisole, and then add Tween-80 to prepare an aqueous ...
Embodiment 2
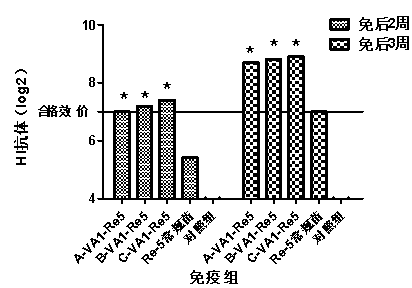
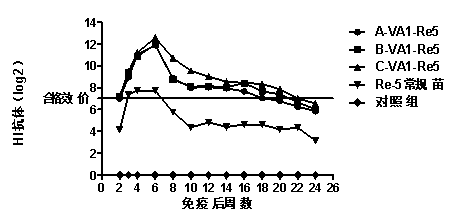
[0053] Example 2 Effect of Compound Immunopotentiator on Immunity Efficacy and Antibody Duration of H5 Subtype Avian Influenza Vaccine
[0054] (1) Test material
[0055] Using the existing H5 subtype avian influenza vaccine, prepare H5 subtype avian influenza vaccines A, B and C containing compound immune enhancer according to the first method of Example 1. H5 subtype avian influenza vaccines A, B and C are referred to as A-VA1-Re5, B-VA1-Re5 and C-VA1-Re5 respectively. H5 subtype avian influenza vaccine and H5 subtype avian influenza standard detection antigen were purchased from Harbin Veken Biotechnology Development Company. White oil for seedling preparation was purchased from Esso, Marcol 52, France, and Tween-80 and Span-80 were purchased from Guangdong Zhaoqing Chaoneng Industrial Co., Ltd. Specific Pathogen Free (SPF) chickens purchased chicken embryos from Beijing Meria Weitong Experimental Animal Technology Co., Ltd., and were reared in isolators after self-hatchi...
Embodiment 3
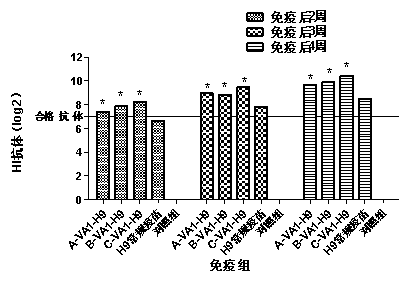
[0068] The impact of embodiment 3 compound immunopotentiator on H9 subtype avian influenza vaccine immune effectiveness
[0069] (1) Test material
[0070] Using the existing H9 subtype avian influenza vaccine, prepare H9 subtype avian influenza vaccines A, B and C containing compound immune enhancer according to the first method of Example 1. The H9 subtype avian influenza vaccines A, B and C containing the compound immune enhancer are referred to as A-VA1-H9, B-VA1-H9 and C-VA1-H9 respectively. The existing H9 subtype avian influenza vaccine and H9 avian influenza detection antigen were purchased from Nanjing Tianbang Biotechnology Co., Ltd.
[0071] (2) Test method
[0072] Immunization and grouping: According to the "Compilation of Quality Standards for Biological Products for Veterinary Use (2006-2008)", referred to as "Procedures", 4-week-old chickens are immunized with 0.3mL / chicken, and chickens over 5 weeks old are immunized with 0.5mL / chicken. The immunization rout...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com